Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels - PubMed (original) (raw)
Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels
Jing Yao et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The molecular basis of the thermal sensitivity of temperature-sensitive channels appears to arise from a specific protein domain rather than integration of global thermal effects. Using systematic chimeric analysis, we show that the N-terminal region that connects ankyrin repeats to the first transmembrane segment is crucial for temperature sensing in heat-activated vanilloid receptor channels. Changing this region both transformed temperature-insensitive isoforms into temperature-sensitive channels and significantly perturbed temperature sensing in temperature-sensitive wild-type channels. Swapping other domains such as the transmembrane core, the C terminus, and the rest of the N terminus had little effect on the steepness of temperature dependence. Our results support that thermal transient receptor potential channels contain modular thermal sensors that confer the unprecedentedly strong temperature dependence to these channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
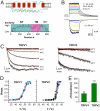
TRPV1 and TRPV2 have distinct temperature dependence. (A) Putative TM topology of vanilloid receptors (Upper) and similarity of TRPV1 and TRPV2 (Lower), plotted as the degree of conservation of residues resulting from sequence alignment by ClustalX2. NT, N terminus; CT, C terminus. (B) Examples of temperature pulses generated by laser irradiation and whole-cell currents evoked by temperature from an HEK 293 cell heterologously expressing rat TRPV1. The temperature pulses, stepped from room temperature (22 °C), were 100 ms long and had a rise time of 0.75 ms. Membrane potential was -60 mV. (C) Fitting of activation time course. To extract temperature dependence, the current traces were fitted to appropriate models (two states for TRPV1 and three states for TRPV2; see text for more details). The dotted lines (red) represent the best fits. (D) Temperature response curves, measured from the maximal currents at the end of temperature steps. Each curve represents measurements from an individual cell. (E) Comparison of temperature dependence of TRPV1 and TRPV2. The activation enthalpy of the endothermic step in the fitted models is shown.
Fig. 2.
Thermal sensitivity involves the membrane-proximal N terminus. (A) Schematic representation of composition of chimeric channels between rat TRPV1 (red) and TRPV2 (gray). The first two chimeras on the top contain the exchange of the entire TM segments. The three chimeras in the middle involve changes in the N terminus. The last two chimeras correspond to TRPV2 with its C-terminal domains replaced by the cognate parts of TRPV1. Only the functional chimeras are shown. Residue numbers correspond to rat TRPV1. (B_–_D) Activation time courses and temperature response curves of chimeric channels (B, TM; C, N terminus; D, C terminus). V1/V2(S1–S6), V2/V1(358–434), and V1/V2(1–283) were fit by a two-state model and others by a three-state model. For comparison, the temperature response curves of wild-type channels are displayed in gray on background. (E) Activation enthalpy of chimeric channels compared with their parental wild-type channels.
Fig. 3.
The membrane-proximal N terminus reveals a conserved mechanism of temperature gating in vanilloid receptors. (A) Sequence alignment of the membrane-proximal N terminus. Gray indicates conserved residues. (B_–_D) Whole-cell currents evoked by temperature for wild-type channels (hTRPV2, mTRPV3, and mTRPV4) and respective chimeras containing rTRPV1-MPD. The exchange of the region restored heat responses in hTRPV2 and mTRPV4 and significantly altered activation properties of mTRPV3. (E) Temperature response curves for chimeras versus wild types. For hTRPV2 and mTRPV4, the current density (normalized by membrane capacitance and then scaled to the mean maximum) is plotted. For mTRPV3, the normalized current is shown. Mean maximum current density (pA/pF): 27 ± 9 (n = 4) for hTRPV2 (WT), 302 ± 55 (n = 6) for hTRPV2/rV1(358–434), 33 ± 4 (n = 3) for mTRPV4 (WT), and 116 ± 20 (n = 3) for mTRPV3/rV1(358–434).
Fig. 4.
Mutations within the membrane-proximal N terminus alter temperature dependence of TRPV1. (A) Amino acid sequence of the membrane-proximal N terminus of rat TRPV1. Five subregions (colored in gray) were mutated, each replaced by the cognate part of TRPV2. The first four replacements resulted in functional channels, whereas the last one was nonfunctional. (B) Representative current traces of mutant channels and their fits by a two-state model. (C) Temperature response curves of mutant channels. Mutations toward the C-terminal half also changed the activation threshold in addition to the slope of the curve. (D) Comparison of activation enthalpy of mutant channels with the wild-type.
Similar articles
- Identification of a helix-turn-helix motif for high temperature dependence of vanilloid receptor TRPV2.
Liu B, Qin F. Liu B, et al. J Physiol. 2021 Nov;599(21):4831-4844. doi: 10.1113/JP282073. Epub 2021 Oct 3. J Physiol. 2021. PMID: 34605028 - ThermoTRP channels as modular proteins with allosteric gating.
Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. Latorre R, et al. Cell Calcium. 2007 Oct-Nov;42(4-5):427-38. doi: 10.1016/j.ceca.2007.04.004. Epub 2007 May 17. Cell Calcium. 2007. PMID: 17499848 Review. - Single-residue molecular switch for high-temperature dependence of vanilloid receptor TRPV3.
Liu B, Qin F. Liu B, et al. Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):1589-1594. doi: 10.1073/pnas.1615304114. Epub 2017 Feb 1. Proc Natl Acad Sci U S A. 2017. PMID: 28154143 Free PMC article. - A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels.
Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. Brauchi S, et al. J Neurosci. 2006 May 3;26(18):4835-40. doi: 10.1523/JNEUROSCI.5080-05.2006. J Neurosci. 2006. PMID: 16672657 Free PMC article. - Gating of thermally activated channels.
Baez D, Raddatz N, Ferreira G, Gonzalez C, Latorre R. Baez D, et al. Curr Top Membr. 2014;74:51-87. doi: 10.1016/B978-0-12-800181-3.00003-8. Curr Top Membr. 2014. PMID: 25366233 Review.
Cited by
- Molecular mechanism of TRP channels.
Zheng J. Zheng J. Compr Physiol. 2013 Jan;3(1):221-42. doi: 10.1002/cphy.c120001. Compr Physiol. 2013. PMID: 23720286 Free PMC article. Review. - Sensing temperature.
Sengupta P, Garrity P. Sengupta P, et al. Curr Biol. 2013 Apr 22;23(8):R304-7. doi: 10.1016/j.cub.2013.03.009. Curr Biol. 2013. PMID: 23618661 Free PMC article. Review. - Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains.
Bagriantsev SN, Clark KA, Minor DL Jr. Bagriantsev SN, et al. EMBO J. 2012 Aug 1;31(15):3297-308. doi: 10.1038/emboj.2012.171. Epub 2012 Jun 22. EMBO J. 2012. PMID: 22728824 Free PMC article. - TRP Channels Role in Pain Associated With Neurodegenerative Diseases.
Duitama M, Vargas-López V, Casas Z, Albarracin SL, Sutachan JJ, Torres YP. Duitama M, et al. Front Neurosci. 2020 Aug 4;14:782. doi: 10.3389/fnins.2020.00782. eCollection 2020. Front Neurosci. 2020. PMID: 32848557 Free PMC article. Review. - Site-specific contacts enable distinct modes of TRPV1 regulation by the potassium channel Kvβ1 subunit.
Wang Y, Mo X, Ping C, Huang Q, Zhang H, Xie C, Zhong B, Li D, Yao J. Wang Y, et al. J Biol Chem. 2020 Dec 11;295(50):17337-17348. doi: 10.1074/jbc.RA120.015605. Epub 2020 Oct 15. J Biol Chem. 2020. PMID: 33060203 Free PMC article.
References
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. - PubMed
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. - PubMed
- Voets T, et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM065994/GM/NIGMS NIH HHS/United States
- R01 GM084891/GM/NIGMS NIH HHS/United States
- R01 GM104521/GM/NIGMS NIH HHS/United States
- R01-GM65994/84891/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources