Planar polarity pathway and Nance-Horan syndrome-like 1b have essential cell-autonomous functions in neuronal migration - PubMed (original) (raw)
Planar polarity pathway and Nance-Horan syndrome-like 1b have essential cell-autonomous functions in neuronal migration
Gregory S Walsh et al. Development. 2011 Jul.
Abstract
Components of the planar cell polarity (PCP) pathway are required for the caudal tangential migration of facial branchiomotor (FBM) neurons, but how PCP signaling regulates this migration is not understood. In a forward genetic screen, we identified a new gene, nhsl1b, required for FBM neuron migration. nhsl1b encodes a WAVE-homology domain-containing protein related to human Nance-Horan syndrome (NHS) protein and Drosophila GUK-holder (Gukh), which have been shown to interact with components of the WAVE regulatory complex that controls cytoskeletal dynamics and with the polarity protein Scribble, respectively. Nhsl1b localizes to FBM neuron membrane protrusions and interacts physically and genetically with Scrib to control FBM neuron migration. Using chimeric analysis, we show that FBM neurons have two modes of migration: one involving interactions between the neurons and their planar-polarized environment, and an alternative, collective mode involving interactions between the neurons themselves. We demonstrate that the first mode of migration requires the cell-autonomous functions of Nhsl1b and the PCP components Scrib and Vangl2 in addition to the non-autonomous functions of Scrib and Vangl2, which serve to polarize the epithelial cells in the environment of the migrating neurons. These results define a role for Nhsl1b as a neuronal effector of PCP signaling and indicate that proper FBM neuron migration is directly controlled by PCP signaling between the epithelium and the migrating neurons.
Figures
Fig. 1.
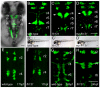
The fh131 mutant disrupts facial branchiomotor (FBM) neuron migration. Confocal images showing dorsal views of the hindbrain of Tg(isl1:GFP)rw0 transgene expression in embryos. Anterior is to the top. (A) Cranial motorneurons are easily visible in whole-mount zebrafish embryos at 48 hours post-fertilization (hpf). Va and Vp, anterior and posterior trigeminal nuclei, respectively, in hindbrain rhombomere (r)2 and r3; VII, facial branchiomotor neurons in r6 with axons exiting the hindbrain in r4; X, vagal motorneurons. (B) Wild-type embryo at 48 hpf with FBM neurons fully migrated into r6 (arrow). (C,D) Zygotic fh131 mutant (B) and maternal-zygotic (mz) fh131 mutant (C) with similarly unmigrated FBM neurons in r4 (arrows). Asterisks mark the cell bodies of the glossopharyngeal (cranial nerve IX) neurons in r7. (B′-D′) Low power transmitted light images of embryos with the genotypes shown in B-D showing otherwise normal morphology at 48 hpf. (E-H) FBM neurons in wild type (E,G) and fh131 mutants (F,H) at the onset of migration at 17 hpf (E,F) and at 24 hpf (G,H) showing that fh131 mutant FBM neurons never leave r4. Scale bars: 50 μm.
Fig. 2.
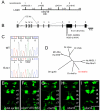
nhsl1b is disrupted in fh131 mutants. (A) Genetic mapping of 1444 fh131 mutant zebrafish embryos identifies a genetic interval on chromosome 20 containing nhsl1b. (B) Genomic structure of nhsl1b. Black boxes mark exons 1-8. (C) Sequence trace of a nonsense mutation in nhsl1b in fh131 mutants. (D) Phylogram of the NHS protein family. Mm, mouse; Dr, zebrafish; Hs, human; Dm, Drosophila. (E,F) Tg(isl1:GFP)rw0 expression in an embryo injected with a splice-blocking morpholino targeted to the nhsl1b exon 4-intron 4 boundary (E) or a translation-blocking morpholino targeted to the ATG of exon 1 (F). (G-I) Tg(isl1:GFP)rw0 expression in PCR-genotyped embryos heterozygous for nhsl1bfh131 (G) or for the nhs1bfh280 nonsense allele generated by TILLING (H) and in nhsl1bfh131/280 trans-heterozygotes (I). Note the strong block to FBM migration in the trans-heterozygotes indicating that fh131 and fh280 are alleles of the same gene. r4-r7, rhombomeres 4-7. Scale bar: 20 μm.
Fig. 3.
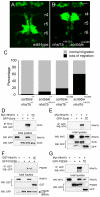
Nhsl1b interacts genetically and physically with Scrib in the regulation of facial branchiomotor (FBM) neuron migration. (A,B) Wild-type (A) and double heterozygous nhsl1bfh131/+; scribrw468/+ zebrafish embryos (B) at 48 hours post-fertilization (hpf). Double heterozygotes have unmigrated FBM neurons in rhombomere (r)4, indicative of a strong genetic interaction between the two genes. (C) Histogram of phenotypes in the genotypic classes arising from a nhsl1bfh131/+ x scribrw468/+ cross. (D-G) Nhsl1b associates with Scrib and Psd95 (Dlg4). cDNA constructs were transfected into HEK293T cells as indicated. Whole cell lysates were immunoprecipitated (IP) and western-blotted (WB) with the indicated antibodies. Scale bar: 20 μm.
Fig. 4.
nhsl1b is expressed in facial branchiomotor (FBM) neurons and localizes to membrane protrusions. (A) RT-PCR from fertilization to 2 days old shows onset of zygotic nhsl1b expression at the end of epiboly [10 hours post-fertilization (hpf)]. ODC, ornithine decarboxylase control. (B-E) mRNA in situ hybridization with nhsl1b in whole mount (B-D) and in cross section at the level of r5 (E) showing widespread, low-level expression in somites (B) and CNS (C) and specific upregulation in cranial motorneurons (D,E). nhsl1b is expressed in FBM neurons in rhombomere (r)4 at the onset of migration (bracket in D) and in r5 and r6 during migration (arrowheads in D, arrow in E) and in trigeminal branchiomotor neurons in r2 (TBM). (F-I′) Whole-mount immunocytochemistry with anti-Nhsl1b (red). Isl1:GFP marks FBM neurons (green). Nhsl1b is localized to the membrane surface of FBM neurons, particularly to membrane protrusions (arrowheads) in wild type (F,G) but not Nhsl1b mutant embryos (H). Nhsl1b is similarly localized in FBM neurons in Scrib mutants (I). I′-J′ show Nhsl1b immunostaining alone. (J) Primary cultures of FBM neurons isolated from Tg(isl1:GFP) fish immunostained for Nhsl1b (red) and GFP (green) shows colocalization of Nhsl1b in motorneurons. (K) Anti-Nhsl1b staining in Tg(isl1CREST-hsp70l:mRFP)fh1 fish showing clear colocalization of Nhsl1b with the cell membrane (arrows). Scale bars: 50 μm for B-E; 7 μm for F-K.
Fig. 5.
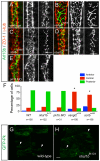
Scrib and Vangl2, but not Nhsl1b or Pk1b, are required for neuroepithelial cell polarity. (A-E) Confocal images showing floorplate planar polarity in 33 hours post-fertilization (hpf) zebrafish embryos. Anterior is to the top. ZO-1 marks subapical tight junctions (red), γ-tubulin marks basal bodies (red, indicated by arrows in A,B) and Arl13b marks the axonemes of primary cilia (green). Whereas basal bodies are localized to the posterior side of floorplate cells in wild type (A), nhsl1bfh131 mutants (D) and pk1b morphants (E) they are centrally located in zygotic vangl2m209 mutants (B) which have a widened floorplate due to defective neural tube convergence and in zygotic scribrw468 mutants (C), which have only a mild neural tube convergence defect. (F) Quantification of the percentage of cells displaying an anterior, central or posterior position of basal bodies in floorplate cells. Asterisk indicates statistically significant difference from wild type (WT) as determined by χ2 test, P<0.0001. (G,H) Live confocal imaging (dorsal view, anterior to the top) of mosaically expressed GFP-Pk marking anterior membranes (arrows) of neuroepithelial progenitors in 16 hpf wild-type (A) and nhsl1bfh131 mutant (B) embryos. Scale bars: 10 μm.
Fig. 6.
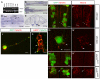
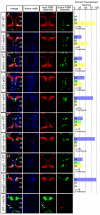
A cell-autonomous role for Nhsl1b, Scrib and Vangl2 in migration. (A-J) Live confocal images at 48 hours post-fertilization (hpf) of chimeric zebrafish embryos with anterior to the top. Cascade blue marks donor-derived cells (blue), Tg(isl1CREST-hsp70l:mRFP)fh1 marks host motorneurons (red) and Tg(isl1:GFP) marks donor-derived motorneurons (green). Histograms on the right indicate the percent of donor-derived FBM neurons in rhombomere (r)4 (unmigrated), r5 and r6 (fully migrated) under the transplantation conditions indicated on the far left, which are written as Donor>Host. n refers to the total number of FBM neurons scored in each condition. Pk1b MOs were used in D, E, G and I to prevent host FBM neurons from migrating by a cell-autonomous mechanism. J shows the rescue of host nhsl1b mutant FBM neurons expressing Tg(isl1:GFP) (green) (arrow) by wild-type donor FBM neurons expressing Tg(isl1CREST-hsp70l:mRFP)fh1 (red). Scale bar: 50 μm.
Fig. 7.
A model for facial branchiomotor (FBM) neuron migration. (A-C) FBM neuron migration requires the planar polarization of both the neurons and the surrounding neuroepithelium. Neurons fail to migrate either owing to lack of neuroepithelial polarity, e.g. in a vangl2 or scrib mutant (B) or owing to the inability of the neurons to be polarized in response to this environment, e.g. in an nhsl1b or pk1b mutant (C). (D,E) Chimeric analysis reveals that FBM neurons can migrate by one of two distinct mechanisms: one which requires the function of PCP proteins both within FBM neurons and the neuroepithelium (D), or collectively, independent of these functions in the ‘rescued’ neurons but requiring the presence of other normally migrated neurons (E). The incomplete migration of donor-derived neurons observed in D or E when only one of these two mechanisms is available indicates that both mechanisms are functioning during normal migration.
Similar articles
- The mouse Wnt/PCP protein Vangl2 is necessary for migration of facial branchiomotor neurons, and functions independently of Dishevelled.
Glasco DM, Sittaramane V, Bryant W, Fritzsch B, Sawant A, Paudyal A, Stewart M, Andre P, Cadete Vilhais-Neto G, Yang Y, Song MR, Murdoch JN, Chandrasekhar A. Glasco DM, et al. Dev Biol. 2012 Sep 15;369(2):211-22. doi: 10.1016/j.ydbio.2012.06.021. Epub 2012 Jul 4. Dev Biol. 2012. PMID: 22771245 Free PMC article. - Multiple mechanisms mediate motor neuron migration in the zebrafish hindbrain.
Bingham SM, Sittaramane V, Mapp O, Patil S, Prince VE, Chandrasekhar A. Bingham SM, et al. Dev Neurobiol. 2010 Feb;70(2):87-99. doi: 10.1002/dneu.20761. Dev Neurobiol. 2010. PMID: 19937772 Free PMC article. - The PCP protein Vangl2 regulates migration of hindbrain motor neurons by acting in floor plate cells, and independently of cilia function.
Sittaramane V, Pan X, Glasco DM, Huang P, Gurung S, Bock A, Li S, Wang H, Kawakami K, Matise MP, Chandrasekhar A. Sittaramane V, et al. Dev Biol. 2013 Oct 15;382(2):400-12. doi: 10.1016/j.ydbio.2013.08.017. Epub 2013 Aug 26. Dev Biol. 2013. PMID: 23988578 Free PMC article. - Facial motor neuron migration advances.
Wanner SJ, Saeger I, Guthrie S, Prince VE. Wanner SJ, et al. Curr Opin Neurobiol. 2013 Dec;23(6):943-50. doi: 10.1016/j.conb.2013.09.001. Epub 2013 Sep 30. Curr Opin Neurobiol. 2013. PMID: 24090878 Free PMC article. Review. - Roles of noncanonical Wnt/PCP pathway genes in neuronal migration and neurulation in zebrafish.
Wada H, Okamoto H. Wada H, et al. Zebrafish. 2009 Mar;6(1):3-8. doi: 10.1089/zeb.2008.0557. Zebrafish. 2009. PMID: 19250033 Review.
Cited by
- Phosphorylation-dependent interaction between tumor suppressors Dlg and Lgl.
Zhu J, Shang Y, Wan Q, Xia Y, Chen J, Du Q, Zhang M. Zhu J, et al. Cell Res. 2014 Apr;24(4):451-63. doi: 10.1038/cr.2014.16. Epub 2014 Feb 11. Cell Res. 2014. PMID: 24513855 Free PMC article. - Shaping the nervous system: role of the core planar cell polarity genes.
Tissir F, Goffinet AM. Tissir F, et al. Nat Rev Neurosci. 2013 Aug;14(8):525-35. doi: 10.1038/nrn3525. Epub 2013 Jul 10. Nat Rev Neurosci. 2013. PMID: 23839596 Review. - SGEF forms a complex with Scribble and Dlg1 and regulates epithelial junctions and contractility.
Awadia S, Huq F, Arnold TR, Goicoechea SM, Sun YJ, Hou T, Kreider-Letterman G, Massimi P, Banks L, Fuentes EJ, Miller AL, Garcia-Mata R. Awadia S, et al. J Cell Biol. 2019 Aug 5;218(8):2699-2725. doi: 10.1083/jcb.201811114. Epub 2019 Jun 27. J Cell Biol. 2019. PMID: 31248911 Free PMC article. - Noncanonical Wnt planar cell polarity signaling in lung development and disease.
Vladar EK, Königshoff M. Vladar EK, et al. Biochem Soc Trans. 2020 Feb 28;48(1):231-243. doi: 10.1042/BST20190597. Biochem Soc Trans. 2020. PMID: 32096543 Free PMC article. Review. - Rest represses maturation within migrating facial branchiomotor neurons.
Love CE, Prince VE. Love CE, et al. Dev Biol. 2015 May 15;401(2):220-35. doi: 10.1016/j.ydbio.2015.02.021. Epub 2015 Mar 11. Dev Biol. 2015. PMID: 25769695 Free PMC article.
References
- Audebert S., Navarro C., Nourry C., Chasserot-Golaz S., Lecine P., Bellaiche Y., Dupont J. L., Premont R. T., Sempere C., Strub J. M., et al. (2004). Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr. Biol. 14, 987-995 - PubMed
- Bahary N., Davidson A., Ransom D., Shepard J., Stern H., Trede N., Zhou Y., Barut B., Zon L. I. (2004). The Zon laboratory guide to positional cloning in zebrafish. Methods Cell Biol. 77, 305-329 - PubMed
- Beattie C. E., Raible D. W., Henion P. D., Eisen J. S. (1999). Early pressure screens. Methods Cell Biol. 60, 71-86 - PubMed
- Bilder D., Perrimon N. (2000). Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676-680 - PubMed
- Bilder D., Li M., Perrimon N. (2000). Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113-116 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases