The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4 - PubMed (original) (raw)
. 2011 Jul 26;50(29):6295-300.
doi: 10.1021/bi200770q. Epub 2011 Jun 29.
Affiliations
- PMID: 21696149
- PMCID: PMC3169095
- DOI: 10.1021/bi200770q
The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4
Chilman Bae et al. Biochemistry. 2011.
Abstract
Cells can respond to mechanical stress by gating mechanosensitive ion channels (MSCs). The cloning of Piezo1, a eukaryotic cation selective MSC, defines a new system for studying mechanical transduction at the cellular level. Because Piezo1 has electrophysiological properties similar to those of endogenous cationic MSCs that are selectively inhibited by the peptide GsMTx4, we tested whether the peptide targets Piezo1 activity. Extracellular GsMTx4 at micromolar concentrations reversibly inhibited ∼80% of the mechanically induced current of outside-out patches from transfected HEK293 cells. The inhibition was voltage insensitive, and as seen with endogenous MSCs, the mirror image d enantiomer inhibited like the l. The rate constants for binding and unbinding based on Piezo1 current kinetics provided association and dissociation rates of 7.0 × 10(5) M(-1) s(-1) and 0.11 s(-1), respectively, and a K(D) of ∼155 nM, similar to values previously reported for endogenous MSCs. Consistent with predicted gating modifier behavior, GsMTx4 produced an ∼30 mmHg rightward shift in the pressure-gating curve and was active on closed channels. In contrast, streptomycin, a nonspecific inhibitor of cationic MSCs, showed the use-dependent inhibition characteristic of open channel block. The peptide did not block currents of the mechanical channel TREK-1 on outside-out patches. Whole-cell Piezo1 currents were also reversibly inhibited by GsMTx4, and although the off rate was nearly identical to that of outside-out patches, differences were observed for the on rate. The ability of GsMTx4 to target the mechanosensitivity of Piezo1 supports the use of this channel in high-throughput screens for pharmacological agents and diagnostic assays.
© 2011 American Chemical Society
Figures
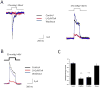
Figure 1
GsMTx4 inhibits Piezo1 currents. Panel A (Left): O-O patch with the baseline response measured at -30 mV with pressure pulses of 500 ms at intervals of 3.5 s (black trace). The mechanical response was inhibited by L-GsMTx4 (2.5 μM, red trace) and washout restored control activity (blue trace). The data are an average of 5-10 pulses. Panel A (Right): A comparison of the -30mV response to the +30mV response shows a mild voltage dependence of inhibition. Panel B demonstrates that the D-enantiomer (3.0 μM) reversibly inhibits Piezo1. Panel C is a summary of the average responses to D (n=3 patches) and L (n=7 patches) enantiomers. Data are normalized to allow comparison between experiments and the error bars are SEM.
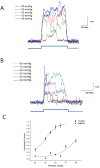
Figure 2
GsMTx4 is a gating inhibitor. O-O patches were stimulated at indicated positive pressure pulses at +50 mV in the absence (Panel A) and in the presence of GsMTx4 (3.0 μM, Panel B). Panel C is a plot of the average peak current fit to a Boltzmann equation (black trace). In the absence of GsMTx4, the midpoint of the gating curve was 48.7 ±1.3 mmHg (SD). In the presence of GsMTx4 P1/2= 76.8 ±2.2 mmHg (SD) (assuming a saturation current equal to that of the control).
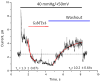
Figure 3
Equilibrium binding constant was determined by association and dissociation kinetics. The indicated pressure pulse was applied to an outside out patch for the lifetime of the experiment. After achieving steady state, a pulse of L-GsMTx4 (2.5 μM) was perfused and inhibition reflected primarily the association rate. Washout of the peptide restored channel activity, reflecting the peptide’s dissociation. Assuming the binding reaction was two states (open-blocked), we extracted the rate constants for association and dissociation from the time constants for wash-in and wash-out using the curve fitting program of QuB (curve fit shown in red, rate constants are indicated with SD). The stippled line is the baseline. GsMTx4 inhibited currents below the baseline indicating that in the “resting patch”, the channels are active, probably as a result of the adhesion energy of the membrane to the glass in the seal. Note that upon the release of the pressure pulse, there is an under shoot of current caused by a transient wrinkling of the membrane. The bowed membrane under pressure has more area than membrane at equilibrium (flat disk). The wrinkled membrane has little tension and that turns off the channels. Over ~1s the membrane reanneals to the glass and restores the resting tension (see reference (18) for a full description of this effect).
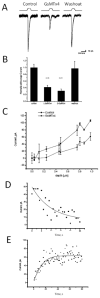
Figure 4
Whole cell currents inhibited by GsMTx4. The cells were indented using the protocol indicated in Panel A. L-GsMTx4 (4.0 μM, red trace) inhibited the mechanosensitive currents (compare to the black trace), and washout returned currents to the original level (Panel A, blue). Notice that GsMTx4 had no effect on the holding current showing that the channels are not active in the resting cell. Panel B, L-GsMTx4 (4.0 μM) inhibited the mechanosensitive currents by 58 ±6% (n=6, S.D.) and the D-GsMTx4 (3.0 μM) inhibited by 70±6% (n=3 S.D.). Panel C demonstrates that whole cell currents increased monotonically with the depth of indentation. Panel D shows the mean dissociation time τd = 10.0 ± 1.7 s (S.D., n=3) was estimated by fitting the recovery time upon washout to a single exponential. This time constant was comparable to that measured for O-O patches. Panel E shows the mean association time constant for 4.0 μM GsMTx4 as τa = 10.4 ± 3.0 s (SD). The rate constant, τd, gave a dissociation rate of _kd_=0.10 s1. However, τa was dominated by kd, and, unlike the rate constant from outside-out patches, the equilibrium constant calculation was untrustworthy.
Similar articles
- Mechanosensitive ion channel Piezo2 is inhibited by D-GsMTx4.
Alcaino C, Knutson K, Gottlieb PA, Farrugia G, Beyder A. Alcaino C, et al. Channels (Austin). 2017 May 4;11(3):245-253. doi: 10.1080/19336950.2017.1279370. Epub 2017 Jan 13. Channels (Austin). 2017. PMID: 28085630 Free PMC article. - GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels.
Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS, Sukharev SI, Suchyna TM. Gnanasambandam R, et al. Biophys J. 2017 Jan 10;112(1):31-45. doi: 10.1016/j.bpj.2016.11.013. Biophys J. 2017. PMID: 28076814 Free PMC article. - Effects of GsMTx4 on bacterial mechanosensitive channels in inside-out patches from giant spheroplasts.
Kamaraju K, Gottlieb PA, Sachs F, Sukharev S. Kamaraju K, et al. Biophys J. 2010 Nov 3;99(9):2870-8. doi: 10.1016/j.bpj.2010.09.022. Biophys J. 2010. PMID: 21044584 Free PMC article. - Piezo1: properties of a cation selective mechanical channel.
Gottlieb PA, Sachs F. Gottlieb PA, et al. Channels (Austin). 2012 Jul-Aug;6(4):214-9. doi: 10.4161/chan.21050. Epub 2012 Jul 1. Channels (Austin). 2012. PMID: 22790400 Free PMC article. Review. - Mechanosensitive ion channels as drug targets.
Gottlieb PA, Suchyna TM, Ostrow LW, Sachs F. Gottlieb PA, et al. Curr Drug Targets CNS Neurol Disord. 2004 Aug;3(4):287-95. doi: 10.2174/1568007043337283. Curr Drug Targets CNS Neurol Disord. 2004. PMID: 15379605 Review.
Cited by
- Mechanosensitive Piezo Channels in Cancer: Focus on altered Calcium Signaling in Cancer Cells and in Tumor Progression.
De Felice D, Alaimo A. De Felice D, et al. Cancers (Basel). 2020 Jul 3;12(7):1780. doi: 10.3390/cancers12071780. Cancers (Basel). 2020. PMID: 32635333 Free PMC article. Review. - Touch sense: functional organization and molecular determinants of mechanosensitive receptors.
Roudaut Y, Lonigro A, Coste B, Hao J, Delmas P, Crest M. Roudaut Y, et al. Channels (Austin). 2012 Jul-Aug;6(4):234-45. doi: 10.4161/chan.22213. Channels (Austin). 2012. PMID: 23146937 Free PMC article. Review. - Mechanosensitivity is an essential component of phototransduction in vertebrate rods.
Bocchero U, Falleroni F, Mortal S, Li Y, Cojoc D, Lamb T, Torre V. Bocchero U, et al. PLoS Biol. 2020 Jul 15;18(7):e3000750. doi: 10.1371/journal.pbio.3000750. eCollection 2020 Jul. PLoS Biol. 2020. PMID: 32667916 Free PMC article. - Piezo1 channel: A global bibliometric analysis from 2010 to 2024.
Tian C, Lyu T, Zhao X, Wang R, Wu Y, Yang D. Tian C, et al. Channels (Austin). 2024 Dec;18(1):2396354. doi: 10.1080/19336950.2024.2396354. Epub 2024 Sep 16. Channels (Austin). 2024. PMID: 39282983 Free PMC article. Review. - The mechanosensitive ion channel Piezo1 modulates the migration and immune response of microglia.
Zhu T, Guo J, Wu Y, Lei T, Zhu J, Chen H, Kala S, Wong KF, Cheung CP, Huang X, Zhao X, Yang M, Sun L. Zhu T, et al. iScience. 2023 Jan 16;26(2):105993. doi: 10.1016/j.isci.2023.105993. eCollection 2023 Feb 17. iScience. 2023. PMID: 36798430 Free PMC article.
References
- Franks NP, Honore E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends in Pharmacological Sciences. 2004;25:601–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources