Crystal structure of clustered regularly interspaced short palindromic repeats (CRISPR)-associated Csn2 protein revealed Ca2+-dependent double-stranded DNA binding activity - PubMed (original) (raw)
Crystal structure of clustered regularly interspaced short palindromic repeats (CRISPR)-associated Csn2 protein revealed Ca2+-dependent double-stranded DNA binding activity
Ki Hyun Nam et al. J Biol Chem. 2011.
Abstract
Clustered regularly interspaced short palindromic repeats (CRISPR) and their associated protein genes (cas genes) are widespread in bacteria and archaea. They form a line of RNA-based immunity to eradicate invading bacteriophages and malicious plasmids. A key molecular event during this process is the acquisition of new spacers into the CRISPR loci to guide the selective degradation of the matching foreign genetic elements. Csn2 is a Nmeni subtype-specific cas gene required for new spacer acquisition. Here we characterize the Enterococcus faecalis Csn2 protein as a double-stranded (ds-) DNA-binding protein and report its 2.7 Å tetrameric ring structure. The inner circle of the Csn2 tetrameric ring is ∼26 Å wide and populated with conserved lysine residues poised for nonspecific interactions with ds-DNA. Each Csn2 protomer contains an α/β domain and an α-helical domain; significant hinge motion was observed between these two domains. Ca(2+) was located at strategic positions in the oligomerization interface. We further showed that removal of Ca(2+) ions altered the oligomerization state of Csn2, which in turn severely decreased its affinity for ds-DNA. In summary, our results provided the first insight into the function of the Csn2 protein in CRISPR adaptation by revealing that it is a ds-DNA-binding protein functioning at the quaternary structure level and regulated by Ca(2+) ions.
Figures
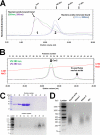
FIGURE 1.
Nucleic acid binding and oligomerization in Csn2. A, elution profile of the Csn2 protein with (peak 1) or without (peak 2) the co-purifying nucleic acids on an analytical Superdex 200 10/300 size exclusion column. Csn2 in peak 2 showed an average size of a pentamer to hexamer formation. Further changes in oligomerization state upon Ca2+ binding are shown in Fig. 5_D. B_, separation of the co-purifying nucleic acids from the Csn2 protein on the Mono Q column. C, analysis of the fractions in panel B using the Coomassie Blue-stained SDS-PAGE gel (upper panel) or ethidium bromide-stained agarose gel (lower panel). Fraction numbers are consistent with those shown in panel B. D, identity of the Csn2-bound nucleic acids examined by incubating with DNase I, RNase A, or the alkaline hydroxyl buffer.
FIGURE 2.
Overall structure of the Csn2 tetrameric ring structure. A, the top-down and the side views (upper and lower panels, respectively) of the Csn2 tetrameric ring structure. The four protomers A–D are colored yellow, cyan, teal, and magenta, respectively. The two 2-fold non-crystallographic symmetry axes and the measurement of the ring dimensions are marked. B, the same views to reveal the electrostatic potential on the Csn2 surface. A clear partition of positive and negative charges to the inner and outer portions of the tetrameric ring, respectively, is observed.
FIGURE 3.
Structural analysis of the Csn2 protomer. A, sequence alignment among the Csn2 family of proteins from E. faecalis (accession code: C7UDU4), Enterococcus faecium (D4W167), L. monocytogenes (E3YJP9), Streptococcus anginosus (E6J3Q7), S. bovis (E0PEL0), Streptococcus pyogenes serotype M1 (Q99ZV9), and S. thermophilus (Q03JI9). The absolutely conserved residues are boxed in red, and the highly conserved ones are in unfilled boxes and red letters. Residues involved in the binding of Ca2+ and ds-DNA (putative) are marked with red and blue asterisks, respectively. B, two views of the monomeric structure of the E. faecalis Csn2 protein. Csn2 consists of an α/β domain and an α-helical domain. Hinge loops between the two domains are colored magenta. A disordered distal loop in the α/β domain is represented by the magenta dotted line. The two Csn2-bound Ca2+ ions are represented in yellow spheres. The two β-sheets and a 310-helix inside the α/β domain are colored in yellow and green, respectively. C, superimposition of the eight Csn2 monomers in the asymmetric unit along the α1-helix (in blue). Hinge motion at the hinge region in magenta leads to ∼5° variation in the orientation of the α-helical domain. Large deviations in equivalent atom positions are marked. D, surface conservation in the Csn2 protomer. Residues are colored from magenta to cyan with descending order of conservation.
FIGURE 4.
Interfaces A-C and A-B that mediate the tetrameric ring formation in the Csn2 protein. A, interface A-C that leads to the dimerization of two α/β domains (colored in yellow and magenta). Side chains of the contacting residues are displayed. B, interface A-B that leads to the dimerization of two β-helical domains. An extensive leucine/isoleucine zipper and four Ca2+-binding sites (two visible) are displayed. C, electrostatic potential of a Csn2 protomer. The interface A-B may be significantly weakened without the Ca2+ ions to shield the strong negative charges at this interface. D, the conserved positive charges from the lysine residues (also in panel C) span a distance of 35 Å along the Csn2 protomer surface. Upon tetramerization, these residues give rise to a positively charged inner surface potentially important for ds-DNA binding.
FIGURE 5.
Ca binding critically influences the oligomerization and DNA binding properties of the Csn2 protein. A, two views of the Ca1- and Ca2-binding sites around the interface A-B. In both cases, Ca2+ binding requires the participation of residues from both Csn2 protomers (in yellow and cyan). B, Ca1 site superimposed with the composite omit electron density contoured at the 5 σ level. Contacting residues from both Csn2 protomers, as well as two ordered water molecules, are highlighted. C, Ca2 site displayed in a similar fashion. Although most Ca2+ chelating residues are from molecule A, Asp-118 from molecule B makes a critical contact to seal this Ca2+-binding site. This contact also rigidifies the conformation of the otherwise flexible loop connecting the α3 and α4 helices in molecule B. D, size exclusion chromatography showing that the presence of Ca2+ ions leads to a more defined Csn2 tetramer formation, whereas the complete removal of Ca2+ ions using EDTA or EGTA leads to a bigger Csn2 oligomer formation. E, EMSA experiments where a titration of Csn2 protein (5–160 μ
m
) was incubated with 100 ng of ds-DNA in the presence of 20 m
m
Ca2+, Mg2+, EDTA, or EGTA. Results showed that the Csn2 protein interacted strongly with the ds-DNA in the presence of Ca2+ ions. This ds-DNA binding activity was decreased to the background level in the presence of Mg2+ or Ca2+-chelating EDTA or EGTA buffers.
Similar articles
- Crystal structure of Streptococcus pyogenes Csn2 reveals calcium-dependent conformational changes in its tertiary and quaternary structure.
Koo Y, Jung DK, Bae E. Koo Y, et al. PLoS One. 2012;7(3):e33401. doi: 10.1371/journal.pone.0033401. Epub 2012 Mar 30. PLoS One. 2012. PMID: 22479393 Free PMC article. - Identification, structural, and biochemical characterization of a group of large Csn2 proteins involved in CRISPR-mediated bacterial immunity.
Lee KH, Lee SG, Eun Lee K, Jeon H, Robinson H, Oh BH. Lee KH, et al. Proteins. 2012 Nov;80(11):2573-82. doi: 10.1002/prot.24138. Epub 2012 Jul 28. Proteins. 2012. PMID: 22753072 - Molecular insights into DNA interference by CRISPR-associated nuclease-helicase Cas3.
Gong B, Shin M, Sun J, Jung CH, Bolt EL, van der Oost J, Kim JS. Gong B, et al. Proc Natl Acad Sci U S A. 2014 Nov 18;111(46):16359-64. doi: 10.1073/pnas.1410806111. Epub 2014 Nov 3. Proc Natl Acad Sci U S A. 2014. PMID: 25368186 Free PMC article. - Clustered regularly interspaced short palindromic repeats (CRISPRs): the hallmark of an ingenious antiviral defense mechanism in prokaryotes.
Al-Attar S, Westra ER, van der Oost J, Brouns SJ. Al-Attar S, et al. Biol Chem. 2011 Apr;392(4):277-89. doi: 10.1515/BC.2011.042. Epub 2011 Feb 7. Biol Chem. 2011. PMID: 21294681 Review. - CRISPR-based adaptive and heritable immunity in prokaryotes.
van der Oost J, Jore MM, Westra ER, Lundgren M, Brouns SJ. van der Oost J, et al. Trends Biochem Sci. 2009 Aug;34(8):401-7. doi: 10.1016/j.tibs.2009.05.002. Epub 2009 Jul 29. Trends Biochem Sci. 2009. PMID: 19646880 Review.
Cited by
- Comparative Genomics Reveals Ecological and Evolutionary Insights into Sponge-Associated Thaumarchaeota.
Zhang S, Song W, Wemheuer B, Reveillaud J, Webster N, Thomas T. Zhang S, et al. mSystems. 2019 Aug 13;4(4):e00288-19. doi: 10.1128/mSystems.00288-19. mSystems. 2019. PMID: 31409660 Free PMC article. - Digging into the lesser-known aspects of CRISPR biology.
Guzmán NM, Esquerra-Ruvira B, Mojica FJM. Guzmán NM, et al. Int Microbiol. 2021 Nov;24(4):473-498. doi: 10.1007/s10123-021-00208-7. Epub 2021 Sep 6. Int Microbiol. 2021. PMID: 34487299 Free PMC article. Review. - Crystal structure of Streptococcus pyogenes Csn2 reveals calcium-dependent conformational changes in its tertiary and quaternary structure.
Koo Y, Jung DK, Bae E. Koo Y, et al. PLoS One. 2012;7(3):e33401. doi: 10.1371/journal.pone.0033401. Epub 2012 Mar 30. PLoS One. 2012. PMID: 22479393 Free PMC article. - CRISPR-Cas systems in enterococci.
Cabral AS, Lacerda FF, Leite VLM, de Miranda FM, da Silva AB, Dos Santos BA, Lima JLDC, Teixeira LM, Neves FPG. Cabral AS, et al. Braz J Microbiol. 2024 Dec;55(4):3945-3957. doi: 10.1007/s42770-024-01549-x. Epub 2024 Oct 23. Braz J Microbiol. 2024. PMID: 39438415 Review. - In silico genomic analysis of the potential probiotic Lactiplantibacillus pentosus CF2-10N reveals promising beneficial effects with health promoting properties.
Abriouel H, Manetsberger J, Caballero Gómez N, Benomar N. Abriouel H, et al. Front Microbiol. 2022 Nov 3;13:989824. doi: 10.3389/fmicb.2022.989824. eCollection 2022. Front Microbiol. 2022. PMID: 36406402 Free PMC article.
References
- Sorek R., Kunin V., Hugenholtz P. (2008) Nat. Rev. Microbiol 6, 181–186 - PubMed
- van der Oost J., Jore M. M., Westra E. R., Lundgren M., Brouns S. J. (2009) Trends Biochem. Sci. 34, 401–407 - PubMed
- Deveau H., Garneau J. E., Moineau S. (2010) Annu. Rev. Microbiol. 64, 475–493 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM-059604/GM/NIGMS NIH HHS/United States
- RR-15301/RR/NCRR NIH HHS/United States
- R01 GM059604/GM/NIGMS NIH HHS/United States
- R01 GM086766/GM/NIGMS NIH HHS/United States
- GM-086766/GM/NIGMS NIH HHS/United States
- P41 RR015301/RR/NCRR NIH HHS/United States
- R01 GM102543/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous