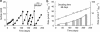Thermophilic anaerobic oxidation of methane by marine microbial consortia - PubMed (original) (raw)
Thermophilic anaerobic oxidation of methane by marine microbial consortia
Thomas Holler et al. ISME J. 2011 Dec.
Abstract
The anaerobic oxidation of methane (AOM) with sulfate controls the emission of the greenhouse gas methane from the ocean floor. AOM is performed by microbial consortia of archaea (ANME) associated with partners related to sulfate-reducing bacteria. In vitro enrichments of AOM were so far only successful at temperatures ≤25 °C; however, energy gain for growth by AOM with sulfate is in principle also possible at higher temperatures. Sequences of 16S rRNA genes and core lipids characteristic for ANME as well as hints of in situ AOM activity were indeed reported for geothermally heated marine environments, yet no direct evidence for thermophilic growth of marine ANME consortia was obtained to date. To study possible thermophilic AOM, we investigated hydrothermally influenced sediment from the Guaymas Basin. In vitro incubations showed activity of sulfate-dependent methane oxidation between 5 and 70 °C with an apparent optimum between 45 and 60 °C. AOM was absent at temperatures ≥75 °C. Long-term enrichment of AOM was fastest at 50 °C, yielding a 13-fold increase of methane-dependent sulfate reduction within 250 days, equivalent to an apparent doubling time of 68 days. The enrichments were dominated by novel ANME-1 consortia, mostly associated with bacterial partners of the deltaproteobacterial HotSeep-1 cluster, a deeply branching phylogenetic group previously found in a butane-amended 60 °C-enrichment culture of Guaymas sediments. The closest relatives (Desulfurella spp.; Hippea maritima) are moderately thermophilic sulfur reducers. Results indicate that AOM and ANME archaea could be of biogeochemical relevance not only in cold to moderate but also in hot marine habitats.
Figures
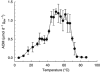
Figure 1
Rates of AOM in Guaymas Basin sediment at different temperatures measured as 14CH4 conversion to 14CO2. Homogenous samples (2–45 cm sediment depth, in situ temperature, 4–85 °C) were pre-incubated for 5 days at designated temperatures followed by incubation with labeled methane for 48 h. Error bars indicate s.d. from triplicates.
Figure 2
Time course experiment of AOM enrichment incubated without headspace at 50 °C. Sulfide formation (black circles) and methane consumption (black triangles) in the enrichment. A control without methane addition (open triangles, background methane) showed only minor sulfide formation (open circles).
Figure 3
Long-term enrichment of anaerobic methanotrophs at 50 °C. (a) Sulfide formation (black circles) in the initial sediment suspension and upon five exchanges of the sulfide-rich supernatant. Medium exchanges are indicated with arrows. (b) Linear (vertical bars) and semi-logarithmic (open circles) illustration of methane-dependent sulfide production of the different incubation periods over time; normalized by dry weights.
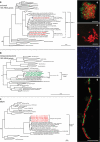
Figure 4
Phylotypes and cell aggregates in the methane-oxidizing anaerobic-enrichment culture grown at 50 °C. Phylogenetic trees showing the affiliations of 16S rRNA gene sequences retrieved from Guaymas methane-oxidizing enrichments with selected reference sequences of (a) Euryarchaeota and (b) Deltaproteobacteria. Sequences from this study are printed in bold red (archaea) and bold green (bacteria). Probe specificity is indicated with brackets. Bar=10% estimated sequence divergence. (c) Phylogenetic tree of amino acid sequences of the α subunit of the methyl-coenzyme M reductase (mcrA), (d–g) cell aggregates of ANME-1 visualized by CARD-FISH. Scale bars=10 μm. (d, e, g) Confocal laser scanning micrographs. (f) Regular epifluorescence micrograph. (d) Spherical ANME-1/HotSeep-1 aggregates stained with probe ANME-1-350 (red) and probe HotSeep-1-590 (green). (e) Monophyletic ANME-1 aggregate stained with probe ANME-1-350. (f) DAPI staining showing long chain-forming ANME-1 aggregates. (g) Chain-forming ANME-1/HotSeep-1 aggregates. ANME-1 cells were identified as members of subcluster ANME-1-Guaymas I (probe ANME-1-GI812, red) and bacterial partners as members of the HotSeep-1 cluster (probe HotSeep-1-590, green).
Similar articles
- Spatial-Temporal Pattern of Sulfate-Dependent Anaerobic Methane Oxidation in an Intertidal Zone of the East China Sea.
Wang J, Hua M, Cai C, Hu J, Wang J, Yang H, Ma F, Qian H, Zheng P, Hu B. Wang J, et al. Appl Environ Microbiol. 2019 Mar 22;85(7):e02638-18. doi: 10.1128/AEM.02638-18. Print 2019 Apr 1. Appl Environ Microbiol. 2019. PMID: 30709818 Free PMC article. - On the relationship between methane production and oxidation by anaerobic methanotrophic communities from cold seeps of the Gulf of Mexico.
Orcutt B, Samarkin V, Boetius A, Joye S. Orcutt B, et al. Environ Microbiol. 2008 May;10(5):1108-17. doi: 10.1111/j.1462-2920.2007.01526.x. Epub 2008 Jan 23. Environ Microbiol. 2008. PMID: 18218032 - Anaerobic oxidation of methane at different temperature regimes in Guaymas Basin hydrothermal sediments.
Biddle JF, Cardman Z, Mendlovitz H, Albert DB, Lloyd KG, Boetius A, Teske A. Biddle JF, et al. ISME J. 2012 May;6(5):1018-31. doi: 10.1038/ismej.2011.164. Epub 2011 Nov 17. ISME J. 2012. PMID: 22094346 Free PMC article. - Physiology and Distribution of Archaeal Methanotrophs That Couple Anaerobic Oxidation of Methane with Sulfate Reduction.
Bhattarai S, Cassarini C, Lens PNL. Bhattarai S, et al. Microbiol Mol Biol Rev. 2019 Jul 31;83(3):e00074-18. doi: 10.1128/MMBR.00074-18. Print 2019 Aug 21. Microbiol Mol Biol Rev. 2019. PMID: 31366606 Free PMC article. Review. - Biogeochemistry and microbial ecology of methane oxidation in anoxic environments: a review.
Valentine DL. Valentine DL. Antonie Van Leeuwenhoek. 2002 Aug;81(1-4):271-82. doi: 10.1023/a:1020587206351. Antonie Van Leeuwenhoek. 2002. PMID: 12448726 Review.
Cited by
- A Reduced F420-Dependent Nitrite Reductase in an Anaerobic Methanotrophic Archaeon.
Heryakusuma C, Susanti D, Yu H, Li Z, Purwantini E, Hettich RL, Orphan VJ, Mukhopadhyay B. Heryakusuma C, et al. J Bacteriol. 2022 Jul 19;204(7):e0007822. doi: 10.1128/jb.00078-22. Epub 2022 Jun 13. J Bacteriol. 2022. PMID: 35695516 Free PMC article. - Harnessing a methane-fueled, sediment-free mixed microbial community for utilization of distributed sources of natural gas.
Marlow JJ, Kumar A, Enalls BC, Reynard LM, Tuross N, Stephanopoulos G, Girguis P. Marlow JJ, et al. Biotechnol Bioeng. 2018 Jun;115(6):1450-1464. doi: 10.1002/bit.26576. Epub 2018 Mar 24. Biotechnol Bioeng. 2018. PMID: 29460958 Free PMC article. - Biomineralization mediated by anaerobic methane-consuming cell consortia.
Chen Y, Li YL, Zhou GT, Li H, Lin YT, Xiao X, Wang FP. Chen Y, et al. Sci Rep. 2014 Jul 16;4:5696. doi: 10.1038/srep05696. Sci Rep. 2014. PMID: 25027246 Free PMC article. - Deep-branching ANME-1c archaea grow at the upper temperature limit of anaerobic oxidation of methane.
Benito Merino D, Zehnle H, Teske A, Wegener G. Benito Merino D, et al. Front Microbiol. 2022 Sep 23;13:988871. doi: 10.3389/fmicb.2022.988871. eCollection 2022. Front Microbiol. 2022. PMID: 36212815 Free PMC article. - A PCR-Based Survey of Methane-Cycling Archaea in Methane-Soaked Subsurface Sediments of Guaymas Basin, Gulf of California.
Hinkle JE, Mara P, Beaudoin DJ, Edgcomb VP, Teske AP. Hinkle JE, et al. Microorganisms. 2023 Dec 10;11(12):2956. doi: 10.3390/microorganisms11122956. Microorganisms. 2023. PMID: 38138100 Free PMC article.
References
- Aeckersberg F, Bak F, Widdel F. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch Microbiol. 1991;156:5–14.
- Aquilina A, Knab NJ, Knittel K, Kaur G, Geissler A, Kelly SP, et al. Biomarker indicators for anaerobic oxidizers of methane in brackish-marine sediments with diffusive methane fluxes. Org Geochem. 2010;41:414–426.
- Boetius A, Holler T, Knittel K, Felden J, Wenzhöfer F.2009The seabed as natural laboratory: lessons from uncultivated methanotrophsIn Epstein SS (ed),Microbiology Monographs: Uncultivated Microorganisms Springer: Berlin; 59–82.
- Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, Gieseke A, et al. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature. 2000;407:623–626. - PubMed
- Bradley AS, Fredricks HF, Hinrichs K-U, Summons RE. Structural diversity of diether lipids in carbonate chimneys at the lost city hydrothermal field. Org Geochem. 2009;40:1169–1178.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous