SIRT1 promotes N-Myc oncogenesis through a positive feedback loop involving the effects of MKP3 and ERK on N-Myc protein stability - PubMed (original) (raw)
. 2011 Jun;7(6):e1002135.
doi: 10.1371/journal.pgen.1002135. Epub 2011 Jun 16.
Pei Y Liu, Samuele Gherardi, Christopher J Scarlett, Antonio Bedalov, Ning Xu, Nuncio Iraci, Emanuele Valli, Dora Ling, Wayne Thomas, Margo van Bekkum, Eric Sekyere, Kacper Jankowski, Toby Trahair, Karen L Mackenzie, Michelle Haber, Murray D Norris, Andrew V Biankin, Giovanni Perini, Tao Liu
Affiliations
- PMID: 21698133
- PMCID: PMC3116909
- DOI: 10.1371/journal.pgen.1002135
SIRT1 promotes N-Myc oncogenesis through a positive feedback loop involving the effects of MKP3 and ERK on N-Myc protein stability
Glenn M Marshall et al. PLoS Genet. 2011 Jun.
Abstract
The N-Myc oncoprotein is a critical factor in neuroblastoma tumorigenesis which requires additional mechanisms converting a low-level to a high-level N-Myc expression. N-Myc protein is stabilized when phosphorylated at Serine 62 by phosphorylated ERK protein. Here we describe a novel positive feedback loop whereby N-Myc directly induced the transcription of the class III histone deacetylase SIRT1, which in turn increased N-Myc protein stability. SIRT1 binds to Myc Box I domain of N-Myc protein to form a novel transcriptional repressor complex at gene promoter of mitogen-activated protein kinase phosphatase 3 (MKP3), leading to transcriptional repression of MKP3, ERK protein phosphorylation, N-Myc protein phosphorylation at Serine 62, and N-Myc protein stabilization. Importantly, SIRT1 was up-regulated, MKP3 down-regulated, in pre-cancerous cells, and preventative treatment with the SIRT1 inhibitor Cambinol reduced tumorigenesis in TH-MYCN transgenic mice. Our data demonstrate the important roles of SIRT1 in N-Myc oncogenesis and SIRT1 inhibitors in the prevention and therapy of N-Myc-induced neuroblastoma.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
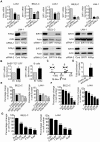
Figure 1. Transcriptional up-regulation of SIRT1 promotes neuroblastoma cell proliferation.
(A) BE(2)-C and LAN-1 neuroblastoma cells were transfected with scrambled control (Cont) siRNA, N-Myc siRNA-1, N-Myc siRNA-2, SIRT1 siRNA-1 or SIRT1 siRNA-2 for 48 hours, followed by RNA and protein extraction, real-time RT-PCR and immunoblot analyses of N-Myc and SIRT1 mRNA and protein expression. (B) Tetracycline (TET) was withdrawn from SHEP TET-OFF cell culture medium to induce N-Myc gene expression, and B-cells were purified from normal mouse bone marrow and transfected with a construct over-expressing full-length N-Myc cDNA or empty vector (EV). SIRT1 gene expression in the cells was analysed by real-time RT-PCR. (C) Schematic representation of the human SIRT1 gene promoter. (D) ChIP assay was performed with control or anti-N-Myc antibody (Ab) and primers targeting amplicon B in BE(2)-C cells. Fold enrichment of SIRT1 gene promoter by the antibodies was calculated by dividing the PCR product from antibody-immunoprecipitated samples by the PCR product from input. (E, F, G) BE(2)-C and LAN-1 cells were transfected with scrambled control siRNA, N-Myc siRNA-1, N-Myc siRNA-2, SIRT1 siRNA-1 or SIRT1 siRNA-2 (E), or treated with vehicle control, Cambinol (F) or Tenovin-6 (G). Seventy-two hours later, relative cell numbers were examined by the Alamar blue assay, and expressed as percentage change in cell number. Error bars represented standard error. * indicated P<0.05, ** P<0.01 and *** P<0.001.
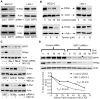
Figure 2. SIRT1 up-regulates N-Myc protein expression by blocking its degradation.
(A, B) BE(2)-C and LAN-1 cells were transfected with scrambled control (Cont) siRNA, SIRT1 siRNA-1 or SIRT1 siRNA-2 (A), or treated with the SIRT1 inhibitor Cambinol, Tenovin-6 or vehicle control (B), followed by protein extraction and immunoblot analysis of N-Myc protein. (C) BE(2)-C cells were transfected with scrambled control siRNA, N-Myc siRNA-1, N-Myc siRNA-2, SIRT1 siRNA-1 or SIRT1 siRNA-2 for 48 hours, followed by treatment with the proteasome inhibitor MG-132 (10 µM) for 3 hours. SIRT1 and N-Myc protein expression was analysed by immunoblot. (D) BE(2)-C cells were transfected with scrambled control siRNA or SIRT1 siRNA-1 for 30 hours, and treated with 50 µM cycloheximide (CHX) for the last 0, 15, 30, 45 or 60 minutes. Protein was extracted from the cells and subjected to immunoblot analysis of N-Myc. N-Myc protein level was normalized by actin, the ratio of N-Myc protein and actin protein was artificially set as 1.0 for samples un-treated with CHX, and half life (T1/2) of N-Myc protein was obtained from the line chart.
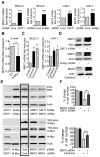
Figure 3. SIRT1 stabilizes N-Myc protein by promoting ERK protein phosphorylation and N-Myc protein phosphorylation at S62.
(A, B) BE(2)-C cells were transfected with scrambled control siRNA, SIRT1 siRNA-1 (left panel) or SIRT1 siRNA-2 (right panel), followed by protein extraction. (A) Expression of total N-Myc protein, N-Myc protein phosphorylated at S62 (S62-phos) and N-Myc protein phosphorylated at T58 (T58-phos) was analysed by immunoblot with specific antibodies. (B) Expression of GSK3 protein, total ERK protein and phosphorylated ERK protein (phos-ERK) was analysed by immunoblot with specific antibodies.
Figure 4. Repression of MKP3 gene expression is required for SIRT1-induced N-Myc protein stabilization and SIRT1-induced cell proliferation.
(A, C, D) BE(2)-C and LAN-1 cells were transfected with scrambled control (Cont), SIRT1 siRNA or N-Myc siRNA (A, D), or treated with vehicle control, 55 µM Cambinol or 5 µM Tenovin-6 (C, D). MKP3 mRNA (A, C) and protein (D) expression was analysed by real-time RT-PCR and immunoblot. (B) B cells from normal mouse bone marrow were transfected with a construct over-expressing full-length N-Myc cDNA or empty vector (EV). MKP3 gene expression was analysed by real-time RT-PCR. (E) BE(2)-C cells were transfected with scrambled control siRNA, MKP3 siRNA, SIRT1 siRNA, N-Myc siRNA, or a combination of MKP3 siRNA and SIRT1 siRNA. Phosphorylated ERK, total ERK, S62-phosphorylated (S62-phos) N-Myc, T58-phosphorylated (T58-phos) N-Myc or total N-Myc protein was examined by immunoblot with specific antibodies. (F) BE(2)-C cells were transfected with scrambled control siRNA, MKP3 siRNA, SIRT1 siRNA, or a combination of MKP3 siRNA and SIRT1 siRNA. In separate experiments, BE(2)-C cells were transfected with scrambled control or MKP3 siRNA and treated with vehicle control or 55 µM Cambinol for 72 hours. Relative total numbers of cells were examined by the Alamar blue assay. Error bars represented standard error. *** indicated P<0.001.
Figure 5. SIRT1 and N-Myc repress MKP3 gene transcription by forming a transcriptional repressor complex at MKP3 gene core promoter.
(A) A schematic representation of the MKP3 gene promoter containing the Sp1 binding sites. (B) Dual cross-linking ChIP and quantitative PCR were applied in BE(2)-C cells. Real-time PCR with primers targeting the negative control region (Amplicon A) or the Sp1-binding sites (Amplicon B) were performed. Fold enrichment of MKP3 promoter regions immunoprecipitated by pre-immune serum (IgG), anti-Sp1, anti-N-Myc and anti-SIRT1 antibodies was calculated as the logarithm of the difference between the cycle-threshold obtained with pre-immune serum and the cycle-threshold obtained with the specific antibody. (C) TET-21/N neuroblastoma cells were transfected with a luciferase reporter construct carrying MKP3 gene promoter region. Luciferase activity of the luciferase reporter construct was determined in the presence (- tetracycline) or absence (+ tetracycline) of N-Myc expression, normalized to that of renilla, and expressed as relative fluorescence units (RFU). Error bars represented standard error. *** indicated P<0.001. (D) HEK 293 cells were transfected with constructs expressing empty vector, N-Myc and/or SIRT1. Nuclear protein from the cells was immunoprecipitated with an anti-N-Myc, anti-SIRT1 or pre-immune serum (IgG) antibody, and co-immunoprecipitation (IP) products were probed with anti-SIRT1 and anti-N-Myc antibodies by immunoblot. (E) Seven different GST-N-Myc deletion mutant expression constructs were generated. GST-N-Myc proteins carrying different moieties of the full length N-Myc were obtained. MB represents Myc Box, and bHLH-zip the basic helix-loop-helix-zipper region. (F) Immobilized GST-N-Myc proteins were loaded with in vitro translated SIRT1 protein. GST-N-Myc complexes were analyzed by immunoblot with an anti-SIRT1 antibody. Amount of loaded GST proteins was also determined by immunoblot.
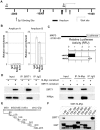
Figure 6. SIRT1 plays an important role in N-Myc–induced neuroblastoma initiation and progression in vivo.
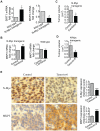
(A, B) Celiac and superior cervical ganglia were dissected from wild type mice and homozygous TH-MYCN transgenic mice at 2 weeks old, and ganglia cells purified. (A) RNA was extracted from the ganglia cells and subjected to real-time RT-PCR analysis of SIRT1 and MKP3 gene expression. SIRT1 and MKP3 expression in ganglia cells from normal mice was artificially set as 1.0. (B) The ganglia cells were treated with vehicle control or 55 µM Cambinol for 24 hours, followed by real-time RT-PCR analysis of MKP3 gene expression. MKP3 gene expression in ganglia cells treated with vehicle control was artificially set as 1.0. (C) Five day old homozygous TH-MYCN transgenic mice were injected intra-peritoneally with Cambinol at the dose of 100 mg/kg/day (number = 8) or vehicle control (number = 8) for 10 consecutive days. They were then left un-treated for 4 weeks, and sacrificed at the age of 42 days. Tumor volume was measured and analyzed. (D) Forty-eight day old homozygous _TH_-MYCN transgenic mice were injected intra-peritoneally with Tenovin-6 at the dose of 50 mg/kg/day (number = 10) or vehicle control (number = 10) for 18 consecutive days. The mice were euthanized at the completion of therapy. Tumor volume was measured and analysed. (E) Neuroblastoma tissues from the _TH_-MYCN transgenic mice treated with control or Tenovin-6 were examined by immunohistochemistry with anti-N-Myc or MKP3 antibodies and visualized with DAB. The nucleus was counter-stained with haematoxylin. N-Myc and MKP3 protein expression was analysed using the scoring system described in Materials and Methods, and expressed as histology score. Error bars indicated standard error. * indicated P<0.05, and ** P<0.01.
Similar articles
- Follicle-Stimulating Hormone (FSH)-dependent Regulation of Extracellular Regulated Kinase (ERK) Phosphorylation by the Mitogen-activated Protein (MAP) Kinase Phosphatase MKP3.
Donaubauer EM, Law NC, Hunzicker-Dunn ME. Donaubauer EM, et al. J Biol Chem. 2016 Sep 9;291(37):19701-12. doi: 10.1074/jbc.M116.733972. Epub 2016 Jul 15. J Biol Chem. 2016. PMID: 27422819 Free PMC article. - The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop.
Menssen A, Hydbring P, Kapelle K, Vervoorts J, Diebold J, Lüscher B, Larsson LG, Hermeking H. Menssen A, et al. Proc Natl Acad Sci U S A. 2012 Jan 24;109(4):E187-96. doi: 10.1073/pnas.1105304109. Epub 2011 Dec 21. Proc Natl Acad Sci U S A. 2012. PMID: 22190494 Free PMC article. - Negative and positive regulation of MAPK phosphatase 3 controls platelet-derived growth factor-induced Erk activation.
Jurek A, Amagasaki K, Gembarska A, Heldin CH, Lennartsson J. Jurek A, et al. J Biol Chem. 2009 Feb 13;284(7):4626-34. doi: 10.1074/jbc.M808490200. Epub 2008 Dec 23. J Biol Chem. 2009. PMID: 19106095 - Feedback regulation of c-Myc by ribosomal protein L11.
Dai MS, Sears R, Lu H. Dai MS, et al. Cell Cycle. 2007 Nov 15;6(22):2735-41. doi: 10.4161/cc.6.22.4895. Epub 2007 Aug 14. Cell Cycle. 2007. PMID: 18032916 Free PMC article. Review. - The SIRT1-c-Myc axis in regulation of stem cells.
Fan W, Li X. Fan W, et al. Front Cell Dev Biol. 2023 Jul 24;11:1236968. doi: 10.3389/fcell.2023.1236968. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37554307 Free PMC article. Review.
Cited by
- Sirtuin functions and modulation: from chemistry to the clinic.
Carafa V, Rotili D, Forgione M, Cuomo F, Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M, Mai A, Altucci L. Carafa V, et al. Clin Epigenetics. 2016 May 25;8:61. doi: 10.1186/s13148-016-0224-3. eCollection 2016. Clin Epigenetics. 2016. PMID: 27226812 Free PMC article. Review. - Ectopic expression of miR-494 inhibited the proliferation, invasion and chemoresistance of pancreatic cancer by regulating SIRT1 and c-Myc.
Liu Y, Li X, Zhu S, Zhang JG, Yang M, Qin Q, Deng SC, Wang B, Tian K, Liu L, Niu Y, Wang CY, Zhao G. Liu Y, et al. Gene Ther. 2015 Sep;22(9):729-38. doi: 10.1038/gt.2015.39. Epub 2015 Apr 28. Gene Ther. 2015. PMID: 25965392 - A molecular circuit linking the BCR to the NAD biosynthetic enzyme NAMPT is an actionable target in Richter syndrome.
Messana VG, Fascì A, Vitale N, Micillo M, Rovere M, Pesce NA, Martines C, Efremov DG, Vaisitti T, Deaglio S. Messana VG, et al. Blood Adv. 2024 Apr 23;8(8):1920-1933. doi: 10.1182/bloodadvances.2023011690. Blood Adv. 2024. PMID: 38359376 Free PMC article. - Dual-Specificity Phosphatases in Neuroblastoma Cell Growth and Differentiation.
Nunes-Xavier CE, Zaldumbide L, Aurtenetxe O, López-Almaraz R, López JI, Pulido R. Nunes-Xavier CE, et al. Int J Mol Sci. 2019 Mar 7;20(5):1170. doi: 10.3390/ijms20051170. Int J Mol Sci. 2019. PMID: 30866462 Free PMC article. Review. - Therapeutic targeting of the MYC signal by inhibition of histone chaperone FACT in neuroblastoma.
Carter DR, Murray J, Cheung BB, Gamble L, Koach J, Tsang J, Sutton S, Kalla H, Syed S, Gifford AJ, Issaeva N, Biktasova A, Atmadibrata B, Sun Y, Sokolowski N, Ling D, Kim PY, Webber H, Clark A, Ruhle M, Liu B, Oberthuer A, Fischer M, Byrne J, Saletta F, Thwe le M, Purmal A, Haderski G, Burkhart C, Speleman F, De Preter K, Beckers A, Ziegler DS, Liu T, Gurova KV, Gudkov AV, Norris MD, Haber M, Marshall GM. Carter DR, et al. Sci Transl Med. 2015 Nov 4;7(312):312ra176. doi: 10.1126/scitranslmed.aab1803. Sci Transl Med. 2015. PMID: 26537256 Free PMC article.
References
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
- Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–2279. - PubMed
- Otto T, Horn S, Brockmann M, Eilers U, Schuttrumpf L, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous