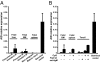Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans - PubMed (original) (raw)
. 2011 Jul 12;108(28):11554-9.
doi: 10.1073/pnas.1102600108. Epub 2011 Jun 23.
Yen-Shing Ng, Jason M Bannock, Aubert Lavoie, Jolan E Walter, Luigi D Notarangelo, Sara S Kilic, Guzide Aksu, Marianne Debré, Frédéric Rieux-Laucat, Mary Ellen Conley, Charlotte Cunningham-Rundles, Anne Durandy, Eric Meffre
Affiliations
- PMID: 21700883
- PMCID: PMC3136251
- DOI: 10.1073/pnas.1102600108
Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans
Greta Meyers et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Impaired immune functions leading to primary immunodeficiencies often correlate with paradoxical autoimmune complications; patients with hyper-IgM syndromes who are deficient in activation-induced cytidine deaminase (AID), which is required for class-switch recombination and somatic hypermutation, are prone to develop autoimmune diseases. To investigate the impact of AID-deficiency on early B-cell tolerance checkpoints in humans, we tested by ELISA the reactivity of recombinant antibodies isolated from single B cells from AID-deficient patients. New emigrant/transitional and mature naive B cells from AID-deficient patients express an abnormal Ig repertoire and high frequencies of autoreactive antibodies, demonstrating that AID is required for the establishment of both central and peripheral B-cell tolerance. In addition, B-cell tolerance was further breached in AID-deficient patients as illustrated by the detection of anti-nuclear IgM antibodies in the serum of all patients. Thus, we identified a major and previously unsuspected role for AID in the removal of developing autoreactive B cells in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
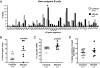
Fig. 1.
AID-deficient new emigrant/transitional B cells display an unusual IgH repertoire. (A) VH gene-usage frequencies in new emigrant/transitional B cells are represented for 11 healthy control subjects and seven AID-deficient patients. Sequences from 353 healthy control and 189 AID-deficient single transitional B cells were pooled. (B) The increased VH4-34 gene usage in AID-deficient new emigrant/transitional B cells is further analyzed. The frequencies of long IgH CDR3s (>14 aa) and IgH CDR3s containing two or more positively charged aa are represented in C and D, respectively. Each diamond represents an individual; the average is shown with a bar. Statistically significant differences are indicated.
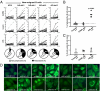
Fig. 2.
Defective central B-cell tolerance checkpoint in AID-deficient patients. (A) Antibodies from new emigrant/transitional B cells from a healthy donor and AID-deficient patients were tested by ELISA for reactivity against dsDNA, insulin, and lipopolysaccharide (LPS). Dotted lines show ED38-positive control and solid lines show binding for each cloned recombinant antibody (20, 26). Horizontal lines define cutoff OD405 for positive reactivity. The frequencies of polyreactive (B) and antinuclear (C) new emigrant/transitional B cells are compared between controls and CD40L- and AID-deficient patients, and statistically significant differences are indicated. (D) Autoreactive antibodies from AID-deficient new emigrant B cells show various patterns of HEp-2 staining.
Fig. 3.
AID gene expression in human immature B cells. AID gene expression was assessed by quantitative PCR in unstimulated human precursors and immature B cells purified from fetal liver, bone marrow (BM), and spleen (A), or in immature B cells stimulated for 2 d with CpG and/or F(ab′)2 anti-IgM (B). Tonsillar GC B cells were used as positive control for AID gene expression. Error bars represent the mean ± SEM.
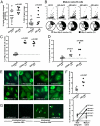
Fig. 4.
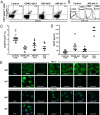
Defective peripheral B-cell tolerance checkpoint in AID-deficient patients. The frequency of mature naive B-cell clones from healthy controls and AID-deficient patients expressing IgH CDR3s containing two or more positively charged aa (Left) or VH4-34 gene (Right) is represented in A, and the average is shown with a bar. (B) Antibodies from mature naive B cells from a healthy donor and AID-deficient patients were tested by ELISA for anti–HEp-2 cell reactivity. Dotted lines show ED38-positive control (20, 26). Horizontal lines define cutoff OD405 for positive reactivity. Frequencies of HEp-2–reactive (C) and polyreactive (D) clones in the mature naive fraction of CD40L and AID-deficient patients are higher than in controls. Mature naive B cells from AID-deficient patients contain ANA-expressing clones with diverse HEp-2 staining patterns (E). The frequency of ANA-expressing B cells in healthy controls and AID-deficient patients (Top) and its evolution between the new emigrant/transitional and mature naive B-cell compartments (Bottom) are represented in F. ANAs that bind the kinetoplast of C. luciliae (white arrows) are shown in G.
Fig. 5.
Low Treg cell frequency and increased serum BAFF concentrations and autoantibodies in AID-deficient patients. Treg cell frequencies among peripheral CD4+ T cells were assessed by analyzing the proportion of CD25+Foxp3+ cells. (A) Dot plots representative of a healthy control, a CD40L-deficient patient, and two AID-deficient patients. (B) CD127 expression was further analyzed by gating as indicated. (C) Treg cell frequencies from all patients were significantly lower than those in healthy controls (P < 0.0001 for CD40L-, AID-deficient, and XLA patients). (D) Significantly elevated serum BAFF concentrations (in pg/mL) in CD40L-, AID-deficient, and XLA patients were measured by ELISA (P < 0.0001 for each group of patients). (E) AID-deficient patients display secreted autoreactive IgM antibodies in their serum. Sera from six healthy donors, a patient with systemic Lupus erythematosus, four CD40L-deficient patients, and eight AID-deficient patients were tested for HEp-2–reactive (Top) and _C. luciliae_-reactive (Bottom) IgM and IgG antibodies.
Similar articles
- Activation-induced cytidine deaminase mediates central tolerance in B cells.
Kuraoka M, Holl TM, Liao D, Womble M, Cain DW, Reynolds AE, Kelsoe G. Kuraoka M, et al. Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11560-5. doi: 10.1073/pnas.1102571108. Epub 2011 Jun 23. Proc Natl Acad Sci U S A. 2011. PMID: 21700885 Free PMC article. - Decreased somatic hypermutation induces an impaired peripheral B cell tolerance checkpoint.
Cantaert T, Schickel JN, Bannock JM, Ng YS, Massad C, Delmotte FR, Yamakawa N, Glauzy S, Chamberlain N, Kinnunen T, Menard L, Lavoie A, Walter JE, Notarangelo LD, Bruneau J, Al-Herz W, Kilic SS, Ochs HD, Cunningham-Rundles C, van der Burg M, Kuijpers TW, Kracker S, Kaneko H, Sekinaka Y, Nonoyama S, Durandy A, Meffre E. Cantaert T, et al. J Clin Invest. 2016 Nov 1;126(11):4289-4302. doi: 10.1172/JCI84645. Epub 2016 Oct 4. J Clin Invest. 2016. PMID: 27701145 Free PMC article. - Activation-Induced Cytidine Deaminase Expression in Human B Cell Precursors Is Essential for Central B Cell Tolerance.
Cantaert T, Schickel JN, Bannock JM, Ng YS, Massad C, Oe T, Wu R, Lavoie A, Walter JE, Notarangelo LD, Al-Herz W, Kilic SS, Ochs HD, Nonoyama S, Durandy A, Meffre E. Cantaert T, et al. Immunity. 2015 Nov 17;43(5):884-95. doi: 10.1016/j.immuni.2015.10.002. Epub 2015 Nov 3. Immunity. 2015. PMID: 26546282 Free PMC article. - Potential roles of activation-induced cytidine deaminase in promotion or prevention of autoimmunity in humans.
Durandy A, Cantaert T, Kracker S, Meffre E. Durandy A, et al. Autoimmunity. 2013 Mar;46(2):148-56. doi: 10.3109/08916934.2012.750299. Epub 2013 Jan 10. Autoimmunity. 2013. PMID: 23215867 Free PMC article. Review. - Hyper-immunoglobulin-M syndromes caused by an intrinsic B cell defect.
Durandy A, Revy P, Fischer A. Durandy A, et al. Curr Opin Allergy Clin Immunol. 2003 Dec;3(6):421-5. doi: 10.1097/00130832-200312000-00002. Curr Opin Allergy Clin Immunol. 2003. PMID: 14612665 Review.
Cited by
- Aberrant B Cell Signaling in Autoimmune Diseases.
Corneth OBJ, Neys SFH, Hendriks RW. Corneth OBJ, et al. Cells. 2022 Oct 27;11(21):3391. doi: 10.3390/cells11213391. Cells. 2022. PMID: 36359789 Free PMC article. Review. - In vivo analysis of Aicda gene regulation: a critical balance between upstream enhancers and intronic silencers governs appropriate expression.
Huong le T, Kobayashi M, Nakata M, Shioi G, Miyachi H, Honjo T, Nagaoka H. Huong le T, et al. PLoS One. 2013 Apr 16;8(4):e61433. doi: 10.1371/journal.pone.0061433. Print 2013. PLoS One. 2013. PMID: 23613851 Free PMC article. - Cellular and molecular mechanisms breaking immune tolerance in inborn errors of immunity.
Sogkas G, Atschekzei F, Adriawan IR, Dubrowinskaja N, Witte T, Schmidt RE. Sogkas G, et al. Cell Mol Immunol. 2021 May;18(5):1122-1140. doi: 10.1038/s41423-020-00626-z. Epub 2021 Apr 1. Cell Mol Immunol. 2021. PMID: 33795850 Free PMC article. Review. - Activation-induced Cytidine Deaminase in B Cell Immunity and Cancers.
Park SR. Park SR. Immune Netw. 2012 Dec;12(6):230-9. doi: 10.4110/in.2012.12.6.230. Epub 2012 Dec 31. Immune Netw. 2012. PMID: 23396757 Free PMC article. - TNF receptor superfamily member 13b (TNFRSF13B) hemizygosity reveals transmembrane activator and CAML interactor haploinsufficiency at later stages of B-cell development.
Romberg N, Virdee M, Chamberlain N, Oe T, Schickel JN, Perkins T, Cantaert T, Rachid R, Rosengren S, Palazzo R, Geha R, Cunningham-Rundles C, Meffre E. Romberg N, et al. J Allergy Clin Immunol. 2015 Nov;136(5):1315-25. doi: 10.1016/j.jaci.2015.05.012. Epub 2015 Jun 19. J Allergy Clin Immunol. 2015. PMID: 26100089 Free PMC article.
References
- Gulino AV, Notarangelo LD. Hyper IgM syndromes. Curr Opin Rheumatol. 2003;15:422–429. - PubMed
- Lee WI, et al. Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome. Blood. 2005;105:1881–1890. - PubMed
- Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. - PubMed
- Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. - PubMed
- Imai K, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI071087/AI/NIAID NIH HHS/United States
- AI071087/AI/NIAID NIH HHS/United States
- P01 AI061093/AI/NIAID NIH HHS/United States
- AI082713/AI/NIAID NIH HHS/United States
- U19 AI082713/AI/NIAID NIH HHS/United States
- R21 AI095848/AI/NIAID NIH HHS/United States
- AI061093/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources