Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice - PubMed (original) (raw)
Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice
David A Hess et al. Gastroenterology. 2011 Oct.
Abstract
Background & aims: Progression of diseases of the exocrine pancreas, which include pancreatitis and cancer, is associated with increased levels of cell stress. Pancreatic acinar cells are involved in development of these diseases and, because of their high level of protein output, they require an efficient, unfolded protein response (UPR) that mediates recovery from endoplasmic reticulum (ER) stress following the accumulation of misfolded proteins.
Methods: To study recovery from ER stress in the exocrine organ, we generated mice with conditional disruption of Xbp1 (a principal component of the UPR) in most adult pancreatic acinar cells (Xbp1fl/fl). We monitored the effects of constitutive ER stress in the exocrine pancreas of these mice.
Results: Xbp1-null acinar cells underwent extensive apoptosis, followed by a rapid phase of recovery in the pancreas that included expansion of the centroacinar cell compartment, formation of tubular complexes that contained Hes1- and Sox9-expressing cells, and regeneration of acinar cells that expressed Mist1 from the residual, surviving Xbp1+ cell population.
Conclusions: XBP1 is required for homeostasis of acinar cells in mice; ER stress induces a regenerative response in the pancreas that involves acinar and centroacinar cells, providing the needed capacity for organ recovery from exocrine pancreas disease.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1. Xbp1fl/fl;Mist1CreER/+ mice selectively ablate Xbp1 in pancreatic acinar cells
(A) Schematic representation of the Xbp1fl/fl and Xbp1ΔEx2 alleles from untreated and tamoxifen-treated mice. (B) Xbp1ΔEx2-specific PCR reveals pancreas-restricted excision of exon 2 following TM treatment (P - pancreas, K - kidney, T - tail DNA). (C) Transcript analysis of the recombined Xbp1ΔEx2 locus confirms Xbp1ΔEx2 transcripts exclusively in pancreatic samples post-TM (Kid. - kidney, Panc. - pancreas RNA). (D) Representative fields of Xbp1fl/fl;Mist1CreER/+;R26RLacZ pancreata treated with or without TM and stained for β-galactosidase (β-gal). Dotted red outline shows a rare β-gal negative acinus. Scale = 40 μm. (E) Quantification of β-gal positive acinar cells in Xbp1fl/fl;Mist1CreER/+;R26R pancreata. *p<0.005
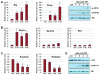
Figure 2. Activation of the UPR following acinar-specific ablation of Xbp1
(A) Relative transcript or protein levels of common ER stress indicators including BiP, Chop, phospho-eIF2α and nuclear ATF6α and (B) Xbp1s following ablation of Xbp1 over the indicated post-TM time points. (B) Xbp1-specific targets Sec61a and PDI do not exhibit increased expression following Xbp1 ablation. (C) Relative transcript levels of amylase and elastase are decreased following Xbp1 ablation. (D) Protein blots revealing decreased production of amylase and increased production of the cleaved, active form of carboxypeptidase (CPAActive), an indicator of intracellular damage to acinar cells. Anti-S6 - loading control. Ctrl - litter mate animals treated with corn oil. *p<0.05
Figure 3. Ablation of Xbp1 leads to severe loss of zymogen granules and alterations in ultrastructure
(A) H&E staining of pancreata 24 hr following Xbp1 ablation. No difference is seen when compared to wild-type pancreata. (B) Pancreas 2 wk after Xbp1 ablation. Reduction in eosinic staining across the pancreas is visible, suggesting significant alterations to the zymogen granule compartment. (C) Pancreas 4 wk post Xbp1 ablation. Greater than 90% of the exocrine pancreas displays little to no eosinic staining. Rare, isolated regions of acinar-like, ZG-bearing cells remain (white outline). (D) Toluidine blue staining of semi-thick sections reveals a completely disrupted exocrine pancreas 4 wk following TM-treatment. Note the rare zymogenic (Z) acinar area (red outline) that remains at this time point. (E) Electron micrograph of a control Xbp1fl/fl; Mist1CreER/+ acinar cell. The extensive accumulation of normal zymogen granules (white arrows) are apically localized. Highly organized rER (yellow arrows and inset) are also visible at the basal edge of the cell. (F) Electron micrograph of a typical non-zymogenic acinar cell from 4 wk post TM-treated Xbp1fl/fl; Mist1CreER/+ mice. Autolysosomes (red arrow), autophagosomes (black arrow), disorganized ER (yellow arrows) and small, abortive zymogen granules (white arrow) can be seen. Inset shows a high mag. image of the extensive free ribosomes found in these cells. Scale = 30 μm (A-D), 1 μm (E-F).
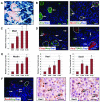
Figure 4. Xbp1ΔEx2 acinar cells extinguish acinar-specific gene expression and undergo ER stress and programmed cell death
(A) β-gal expression in Xbp1fl/fl;Mist1CreER/+;R26RLacZ pancreata 4 wk post-TM. The majority of acinar cells are non-zymogenic β-gal positive although isolated, zymogen-containing clusters of acinar cells (outlines) remain β-gal negative. (B) Zymogen-containing regions (arrows) accumulate high levels of amylase and MIST1, a transcription factor linked to terminal differentiation of the exocrine pancreas. (C) Relative transcript levels of Mist1 reveals an increase in expression following Xbp1 ablation despite restriction to only the zymogenic cell population. (D) Localization of the ER stress indicator CHOP and TUNEL-positive cells (arrows) is largely restricted to non-zymogenic cells. Dotted outlines - individual zymogenic-containing acinar units. (E) Relative transcript levels of pancreatic progenitor cell genes nestin, Hes1 and Sox9 following Xbp1 ablation. (F) Expansion of the centroacinar/terminal duct cell compartment is detected by increased numbers of Hes1 and Sox9 positive cells (arrows) which are amylase negative. Brightfield IHC Hes1, Sox9 - serial sections. Scale = 20 μm. *p<0.05
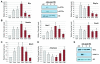
Figure 5. Xbp1ΔEx2 pancreata undergo a rapid recovery period following peak ER stress and apoptosis responses
(A) Relative transcript and protein levels of the ER stress markers BiP, p-eIF2α, nATF6α and Xbp1s are reduced to near control levels over the 4-6 wk post-TM period. (B) Relative transcript levels of pancreatic progenitor cell genes Nestin, Hes1 and Sox9. (C,D) Expression of acinar cell terminal differentiation markers Mist1, Amylase and Elastase return to control levels by 6 wk post-TM. (E) Amylase and activated CPA (CPAActive) protein levels return to control levels over the duration of the time course. Anti-S6 - loading control. Ctrl - litter mate animals treated with corn oil. *p<0.05
Figure 6. The zymogen-containing subset of acinar cells rapidly proliferates and regenerates the exocrine pancreas
(A) Zymogen-containing populations (red outline) are highly proliferative as evidenced by expression of Ki67 and phospho-histone3 (pH3) (arrows). All non-zymogenic acinar cells remain Ki67 and pH3 negative. (B) The Sox9 centroacinar/terminal duct cell compartment is also highly proliferative. (C) Anti-β-gal of 4 wk and 8 wk Xbp1ΔEx2 pancreata showing that the vast majority of acinar cells at 4 wk post-TM are β-gal+. By 8 wk post-TM the exocrine pancreas consists almost entirely of β-gal negative acinar cells. Dotted outline highlights a zymogen-containing acinus. (D) Xbp1ΔEx2 transcript levels over the indicated time points following TM addition. As predicted, replacement of Xbp1ΔEx2 acinar cells with Xbp1fl/fl acinar cells leads to a loss of the Xbp1ΔEx2 allele. (E) H&E staining reveals the rapid recovery of the acinar cell population by 6 wk post-Xbp1 ablation. (F) Toluidine blue staining of semi-thick sections shows the presence of large numbers of zymogen-containing acini (Z) 8 wk post-TM. Zymogen granules are detected as the dark blue staining in the center of the cells. The recovered pancreas also exhibits several other characteristics including the presence of tubular duct-like structures (black arrows), occasional non-zymogenic areas (red outlines) and fat deposition. Note that the tubular complexes are Sox9 positive, suggesting that they are derived from the expanding centroacinar/terminal duct cell compartment. D - duct, Ac - acinar cells. Scale = 20 μm. *p<0.05
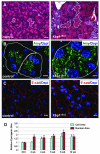
Figure 7. Recovered acini have an expanded zymogen compartment and overall increased cell size relative to control pancreata
(A) Comparison between eosin-stained zymogen compartments in control vs. 12 wk post-ablation pancreata. White dotted lines highlight apically localized zymogens from individual acini. (B) Amylase staining in control and 12 wk Xbp1ΔEx2 pancreata reveal a greatly expanded zymogen compartment in the regenerating acinar cells. Outlines indicate individual acini. (C) E-cadherin staining highlights the overall size of individual acinar cells of control and 12 wk post-TM pancreata showing an increased size for the regenerated cells. (D) Quantification of nuclear and acinar cell size for control and Xbp1ΔEx2 pancreata. Scale = 20 μm. *p<0.005
Comment in
- Pancreatic stress and regeneration.
DiMagliano MP. DiMagliano MP. Gastroenterology. 2011 Oct;141(4):1155-8. doi: 10.1053/j.gastro.2011.08.024. Epub 2011 Aug 24. Gastroenterology. 2011. PMID: 21871457 No abstract available.
Similar articles
- Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage.
Lugea A, Tischler D, Nguyen J, Gong J, Gukovsky I, French SW, Gorelick FS, Pandol SJ. Lugea A, et al. Gastroenterology. 2011 Mar;140(3):987-97. doi: 10.1053/j.gastro.2010.11.038. Epub 2010 Nov 25. Gastroenterology. 2011. PMID: 21111739 Free PMC article. - Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis.
Kowalik AS, Johnson CL, Chadi SA, Weston JY, Fazio EN, Pin CL. Kowalik AS, et al. Am J Physiol Gastrointest Liver Physiol. 2007 Apr;292(4):G1123-32. doi: 10.1152/ajpgi.00512.2006. Epub 2006 Dec 14. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17170023 - Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells.
Kubisch CH, Logsdon CD. Kubisch CH, et al. Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292(6):G1804-12. doi: 10.1152/ajpgi.00078.2007. Epub 2007 Apr 12. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17431218 - Targeting the IRE1α-XBP1 branch of the unfolded protein response in human diseases.
Jiang D, Niwa M, Koong AC. Jiang D, et al. Semin Cancer Biol. 2015 Aug;33:48-56. doi: 10.1016/j.semcancer.2015.04.010. Epub 2015 May 16. Semin Cancer Biol. 2015. PMID: 25986851 Free PMC article. Review. - Alcohol abuse, endoplasmic reticulum stress and pancreatitis.
Pandol SJ, Gorelick FS, Gerloff A, Lugea A. Pandol SJ, et al. Dig Dis. 2010;28(6):776-82. doi: 10.1159/000327212. Epub 2011 Apr 27. Dig Dis. 2010. PMID: 21525762 Free PMC article. Review.
Cited by
- Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice.
Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Collins MA, et al. J Clin Invest. 2012 Feb;122(2):639-53. doi: 10.1172/JCI59227. Epub 2012 Jan 9. J Clin Invest. 2012. PMID: 22232209 Free PMC article. - Induced PTF1a expression in pancreatic ductal adenocarcinoma cells activates acinar gene networks, reduces tumorigenic properties, and sensitizes cells to gemcitabine treatment.
Jakubison BL, Schweickert PG, Moser SE, Yang Y, Gao H, Scully K, Itkin-Ansari P, Liu Y, Konieczny SF. Jakubison BL, et al. Mol Oncol. 2018 Jun;12(7):1104-1124. doi: 10.1002/1878-0261.12314. Epub 2018 May 21. Mol Oncol. 2018. PMID: 29719936 Free PMC article. - Engineering of a functional pancreatic acinus with reprogrammed cancer cells by induced PTF1a expression.
Venis SM, Moon HR, Yang Y, Utturkar SM, Konieczny SF, Han B. Venis SM, et al. Lab Chip. 2021 Sep 28;21(19):3675-3685. doi: 10.1039/d1lc00350j. Lab Chip. 2021. PMID: 34581719 Free PMC article. - Transcriptional Profile of Human Pancreatic Acinar Ductal Metaplasia.
Jiang J, Hakimjavadi H, Bray JK, Perkins C, Gosling A, daSilva L, Bulut G, Ali J, Setiawan VW, Campbell-Thompson M, Chamala S, Schmittgen TD. Jiang J, et al. Gastro Hep Adv. 2023;2(4):532-543. doi: 10.1016/j.gastha.2023.02.003. Epub 2023 Feb 8. Gastro Hep Adv. 2023. PMID: 37425649 Free PMC article. - Heteroclitic XBP1 peptides evoke tumor-specific memory cytotoxic T lymphocytes against breast cancer, colon cancer, and pancreatic cancer cells.
Bae J, Samur M, Munshi A, Hideshima T, Keskin D, Kimmelman A, Lee AH, Dranoff G, Anderson KC, Munshi NC. Bae J, et al. Oncoimmunology. 2014 Dec 2;3(12):e970914. doi: 10.4161/21624011.2014.970914. eCollection 2014. Oncoimmunology. 2014. PMID: 25941601 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- AI32412/AI/NIAID NIH HHS/United States
- R01 DK055489-12/DK/NIDDK NIH HHS/United States
- DK55489/DK/NIDDK NIH HHS/United States
- R01 AI032412/AI/NIAID NIH HHS/United States
- R01 CA124586-03/CA/NCI NIH HHS/United States
- R01 DK055489/DK/NIDDK NIH HHS/United States
- R01 CA124586/CA/NCI NIH HHS/United States
- CA124586/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials