PKCα mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells - PubMed (original) (raw)
PKCα mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells
Ravi K Adapala et al. Am J Physiol Heart Circ Physiol. 2011 Sep.
Abstract
Transient receptor potential vanilloid channel 4 (TRPV4) is a polymodally activated nonselective cationic channel implicated in the regulation of vasodilation and hypertension. We and others have recently shown that cyclic stretch and shear stress activate TRPV4-mediated calcium influx in endothelial cells (EC). In addition to the mechanical forces, acetylcholine (ACh) was shown to activate TRPV4-mediated calcium influx in endothelial cells, which is important for nitric oxide-dependent vasodilation. However, the molecular mechanism through which ACh activates TRPV4 is not known. Here, we show that ACh-induced calcium influx and endothelial nitric oxide synthase (eNOS) phosphorylation but not calcium release from intracellular stores is inhibited by a specific TRPV4 antagonist, AB-159908. Importantly, activation of store-operated calcium influx was not altered in the TRPV4 null EC, suggesting that TRPV4-dependent calcium influx is mediated through a receptor-operated pathway. Furthermore, we found that ACh treatment activated protein kinase C (PKC) α, and inhibition of PKCα activity by the specific inhibitor Go-6976, or expression of a kinase-dead mutant of PKCα but not PKCε or downregulation of PKCα expression by chronic 12-O-tetradecanoylphorbol-13-acetate treatment, completely abolished ACh-induced calcium influx. Finally, we found that ACh-induced vasodilation was inhibited by the PKCα inhibitor Go-6976 in small mesenteric arteries from wild-type mice, but not in TRPV4 null mice. Taken together, these findings demonstrate, for the first time, that a specific isoform of PKC, PKCα, mediates agonist-induced receptor-mediated TRPV4 activation in endothelial cells.
Figures
Fig. 1.
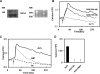
Acetylcholine (ACh) induces transient receptor potential vanilloid channel 4 (TRPV4)-dependent rapid calcium signal in endothelial cells. A: Western blot and RT-PCR analysis showing the expression of TRPV4 protein and message in mouse endothelial cells (MEC). MW, mol wt. B: calcium transients showing that a specific activator of TRPV4, GSK-1016790 (GSK, 100 and 300 nM), induced calcium influx in MEC that is abolished in the absence of calcium (Ca2+ 0) in the media. Arrow indicates addition of the stimulator. F/F0, ratio of fluorescense. C: representative traces showing calcium influx induced by 10 μM of ACh in the MEC in the presence of the muscarinic ACh receptor antagonist atropine (At) or the specific TRPV4 antagonist AB-159908 (AB1). Note that TRPV4 antagonist greatly attenuated the sustained phase (influx) of the calcium signal. D: quantitative analysis of relative changes in calcium influx (by measuring the fluo 4 fluorescence at 120 s) in MEC in the presence or absence of the above-indicated inhibitors. The results shown are means ± SE from 3 independent experiments (*P < 0.05).
Fig. 2.
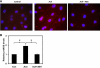
TRPV4-mediated calcium signal is required for endothelial nitric oxide synthase (eNOS) activation by ACh. A: fluorescent images of endothelial cells showing eNOS phosphorylation (p) by ACh. MEC were stimulated with ACh for 15 min in the presence or absence of the TRPV4 antagonist AB-159908. eNOS phosphorylation was measured by incubation with the phosphospecific antibody Ser1177 followed by Alexa-594-conjugated secondary antibody (red) and DAPI (blue). B: quantitative analysis of the eNOS phosphorylation. The results shown are means ± SE from 3 independent experiments (*P < 0.05).
Fig. 3.
TRPV4 channels mediate ACh-induced calcium influx but not release from the internal stores. A: MEC were loaded with fluo 4, and calcium release (first peak) was measured in calcium-free media in response to ACh. The calcium influx (second peak) was initiated by the addition of calcium chloride (2 mM final concentration) to the media. Note that the TRPV4 antagonist AB-155908 reduced the calcium influx but not the release of calcium from the internal stores. Arrow indicates addition of the stimulator. B: quantitative analysis of the calcium influx. The results shown are means ± SE from 3 independent experiments (*P < 0.05).
Fig. 4.
A: RT-PCR analysis showing the expression of the endothelial marker platelet endothelial cell adhesion molecule (PECAM)-1 and the absence of TRPV4 in endothelial cells isolated from TRPV4 null [knockout (KO)] mice. The wild type (WT) and MEC express both PECAM-1 and TRPV4. B: calcium influx is absent in endothelial cells isolated from TRPV4 KO mice (KOEC) stimulated with the specific TRPV4 activator GSK-1016790A (GSK). WTEC, wild-type endothelial cells. Arrow indicates addition of the stimulator. C: quantitative analysis of the calcium influx (*P < 0.05). D: representative traces showing calcium influx measured by using a calcium-free protocol in WT endothelial cells in response to ACh. Arrow indicates addition of the stimulator.
Fig. 5.
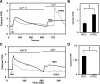
ACh-induced TRPV4-dependent calcium influx is mediated by a receptor-operated pathway. Representative traces showing calcium influx measured by using a calcium-free protocol in MEC and TRPV4 null endothelial cells (KOEC) in response to thapsigargin (TG; A) or ACh (C). Arrow indicates addition of the stimulator. Note that the calcium influx induced by ACh but not TG is inhibited in KOEC, indicating that TRPV4-dependent calcium influx is mediated through a receptor-operated pathway. Quantitative analysis of the calcium influx from the store-operated pathway induced by TG (B) or the receptor-operated pathway induced by ACh (D) in MEC and KOEC. The results shown are means ± SE from 3 independent experiments (*P < 0.05).
Fig. 6.
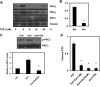
Protein kinase C (PKC) α activity is required for TRPV4-mediated calcium influx by ACh. Representative Western blot showing the downregulation of the expression of PKCα (A) but not PKCε or PKCγ in MEC by chronic 12-_O_-tetradecanoylphorbol-13-acetate (TPA, 1 μM) treatment. B: quantitative analysis of the downregulation of PKCα by TPA. C: representative Western blot showing the activation of PKCα by ACh, which is inhibited by the PKCα specific inhibitor Go-6976 (Go). con, Control. Bar graph shows quantitative analysis of PKCα activation. D: quantitative analysis of the calcium influx induced by ACh in MEC either treated with chronic TPA for 8–12 h (ACh + TPA) or the specific PKCα inhibitor Go-6976 at two concentrations (ACh + Go 1 μM and ACh + Go 10 μM). The results shown are means ± SE from 3 independent experiments (*P < 0.05).
Fig. 7.
Expression of a kinase-dead PKCα inhibits ACh-induced calcium influx. A: representative traces showing calcium influx measured by using the calcium-free protocol in MEC transfected with PKCα-kinase dead (KD)-enhanced green fluorescent protein (EGFP) or PKCε-KD-EGFP expression plasmid in response to ACh. Note that the calcium influx induced by ACh is inhibited in PKCα-KD-expressing cells but not in PKCε-KD-expressing cells. Arrow indicates addition of the stimulator. B: quantitative analysis of the calcium influx induced by ACh in MEC-expressing PKCα-KD-EGFP or PKCε-KD-EGFP (inset shows EGFP fluorescence, confirming the expression of kinase-dead constructs).The results shown are means ± SE from 3 independent experiments (*P < 0.05).
Fig. 8.
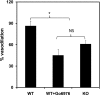
Inhibition of PKCα activity reduces ACh-induced vasodilation in situ in isolated vessels. Quantitative analysis of the vasodilation induced by ACh in mesenteric arteries from WT C57BL6 in the absence or the presence of the specific PKCα inhibitor Go-6976 (WT + Go-6976) and TRPV4 null mice (KO) (n = 5 vessels; *P < 0.05). NS, not significant.
Similar articles
- LAV-BPIFB4 isoform modulates eNOS signalling through Ca2+/PKC-alpha-dependent mechanism.
Spinelli CC, Carrizzo A, Ferrario A, Villa F, Damato A, Ambrosio M, Madonna M, Frati G, Fucile S, Sciaccaluga M, Capunzo M, Calì G, Milanesi L, Maciag A, Puca AA, Vecchione C. Spinelli CC, et al. Cardiovasc Res. 2017 Jun 1;113(7):795-804. doi: 10.1093/cvr/cvx072. Cardiovasc Res. 2017. PMID: 28419216 Free PMC article. - Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo.
Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Zhang DX, et al. Hypertension. 2009 Mar;53(3):532-8. doi: 10.1161/HYPERTENSIONAHA.108.127100. Epub 2009 Feb 2. Hypertension. 2009. PMID: 19188524 Free PMC article. - Omega-3 fatty acids improve flow-induced vasodilation by enhancing TRPV4 in arteries from diet-induced obese mice.
Zhu Y, Wen L, Wang S, Zhang K, Cui Y, Zhang C, Feng L, Yu F, Chen Y, Wang R, Ma X. Zhu Y, et al. Cardiovasc Res. 2021 Nov 1;117(12):2450-2458. doi: 10.1093/cvr/cvaa296. Cardiovasc Res. 2021. PMID: 33070195 - Role of TRPV4 channel in vasodilation and neovascularization.
Chen M, Li X. Chen M, et al. Microcirculation. 2021 Aug;28(6):e12703. doi: 10.1111/micc.12703. Epub 2021 May 24. Microcirculation. 2021. PMID: 33971061 Review. - Endothelial TRPV4 channels and vasodilator reactivity.
Chen YL, Sonkusare SK. Chen YL, et al. Curr Top Membr. 2020;85:89-117. doi: 10.1016/bs.ctm.2020.01.007. Epub 2020 Feb 12. Curr Top Membr. 2020. PMID: 32402646 Free PMC article. Review.
Cited by
- Prenatal stress induces changes in PAR2- and M3-dependent regulation of colon primitive cells.
Berger M, Guiraud L, Dumas A, Sagnat D, Payros G, Rolland C, Vergnolle N, Deraison C, Cenac N, Racaud-Sultan C. Berger M, et al. Am J Physiol Gastrointest Liver Physiol. 2022 Dec 1;323(6):G609-G626. doi: 10.1152/ajpgi.00061.2022. Epub 2022 Oct 25. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 36283083 Free PMC article. - TRPV4 Mechanotransduction in Fibrosis.
Adapala RK, Katari V, Teegala LR, Thodeti S, Paruchuri S, Thodeti CK. Adapala RK, et al. Cells. 2021 Nov 6;10(11):3053. doi: 10.3390/cells10113053. Cells. 2021. PMID: 34831281 Free PMC article. Review. - TRPV4: a Sensor for Homeostasis and Pathological Events in the CNS.
Kumar H, Lee SH, Kim KT, Zeng X, Han I. Kumar H, et al. Mol Neurobiol. 2018 Nov;55(11):8695-8708. doi: 10.1007/s12035-018-0998-8. Epub 2018 Mar 26. Mol Neurobiol. 2018. PMID: 29582401 Review. - P2Y1 Receptor Activation of the TRPV4 Ion Channel Enhances Purinergic Signaling in Satellite Glial Cells.
Rajasekhar P, Poole DP, Liedtke W, Bunnett NW, Veldhuis NA. Rajasekhar P, et al. J Biol Chem. 2015 Nov 27;290(48):29051-62. doi: 10.1074/jbc.M115.689729. Epub 2015 Oct 16. J Biol Chem. 2015. PMID: 26475857 Free PMC article. - Mechanosensitive transient receptor potential vanilloid 4 regulates _Dermatophagoides farinae_-induced airway remodeling via 2 distinct pathways modulating matrix synthesis and degradation.
Gombedza F, Kondeti V, Al-Azzam N, Koppes S, Duah E, Patil P, Hexter M, Phillips D, Thodeti CK, Paruchuri S. Gombedza F, et al. FASEB J. 2017 Apr;31(4):1556-1570. doi: 10.1096/fj.201601045R. Epub 2017 Jan 10. FASEB J. 2017. PMID: 28073835 Free PMC article.
References
- Becker D, Muller M, Leuner K, Jendrach M. The C-terminal domain of TRPV4 is essential for plasma membrane localization. Mol Membr Biol 25: 139–151, 2008 - PubMed
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 23: 617–624, 1999 - PubMed
- Drexler H, Hornig B. Endothelial dysfunction in human disease. J Mol Cell Cardiol 31: 51–60, 1999 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases