The circadian clock of Neurospora crassa - PubMed (original) (raw)
Review
The circadian clock of Neurospora crassa
Christopher L Baker et al. FEMS Microbiol Rev. 2012 Jan.
Abstract
Circadian clocks organize our inner physiology with respect to the external world, providing life with the ability to anticipate and thereby better prepare for major fluctuations in its environment. Circadian systems are widely represented in nearly all major branches of life, except archaebacteria, and within the eukaryotes, the filamentous fungus Neurospora crassa has served for nearly half a century as a durable model organism for uncovering the basic circadian physiology and molecular biology. Studies using Neurospora have clarified our fundamental understanding of the clock as nested positive and negative feedback loops regulated through transcriptional and post-transcriptional processes. These feedback loops are centered on a limited number of proteins that form molecular complexes, and their regulation provides a physical explanation for nearly all clock properties. This review will introduce the basics of circadian rhythms, the model filamentous fungus N. crassa, and provide an overview of the molecular components and regulation of the circadian clock.
© 2011 Federation of European Microbiological Societies. Published by Blackwell Publishing Ltd. All rights reserved.
Figures
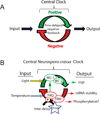
Figure 1. Oscillator model for the circadian clock
(A) Circadian systems comprise three essential elements: an endogenous self-sustaining oscillator, an ability to sense environmental time cues (Input), and physiological output tied to the oscillator at distinct phases. Environmental variables such as light and temperature can entrain or couple the core clock via input pathways. The central oscillator is based on the interplay between positive acting factors driving the expression of negative factors which feedback to inhibit the positive complex. A critical component to this feedback is a mechanism for time delay of the negative state variable that reflects the ~24 hour nature of the physiological rhythms. (B) The organization of molecular components of the feedback loop in Neurospora crassa. The entraining variables of light and temperature impact different parts of the oscillator, light acting through the photoreceptor function of WC-1 to induce transcription of frq and temperature acting to modulate amounts of FRQ. WC-1 and WC-2 form the WCC complex. FRQ and FRH form the FFC complex. Rhythmic output is primarily generated through rhythmic expression of clock-controlled genes (ccgs) but can also be due to changes in mRNA stability and possibly phosphorylation. Kinases contribute to the time-delay by influencing the stability of FRQ.
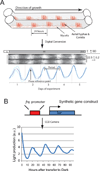
Figure 2. Recording and analysis of rhythms
(A) Race tube assay - glass tubes containing growth media are inoculated at one end and placed in constant light (LL) for one day prior to shifting to constant darkness (DD) which synchronizes the clocks in the culture by setting them all to subjective dusk. Mycelia then ‘race’ down the tube growing at a semi-constant rate such that distance grown approximates time since the light to dark transfer in a linear fashion. Production of the asexual conidia is gated in a daily, phase-specific manner by the circadian clock. These bands of conidia provide a self-reporting time-history of development. This information can be digitized and densitometric analysis of the images result in raw numerical data. Peak of conidial production is often defined as the phase reference point allowing calculation of the period with high precision. (τ = mean period calculation, SD = standard deviation, n = number of tubes for which period was determined). (B) Synthetic engineering of the firefly luciferase enzyme connected to the frq promoter provides a highly quantitative, automated measurement of rhythmic gene expression readily adaptable to high throughput methods. Recording of rhythms of the central FRQ oscillator via luciferase activity also allows investigators to make a distinction between circadian regulation within the core clock and circadian or other regulation of growth wherein different metabolic rhythms might control conidiation and mask circadian output. This system can be adopted to follow regulation of any ccg.
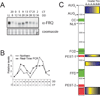
Figure 3. FREQUENCY, a central molecular component in the Neurospora crassa circadian clock
(A) Representative Western blot of FRQ to visualize dynamic protein levels and phosphorylation. Each lane contains equal portions of protein extracted from cultures grown in constant darkness (DD) over one circadian day and harvested at four-hour intervals (LL represents cultures from constant light). FRQ undergoes dual molecular rhythms in protein abundance and phosphorylation peaking in the late subjective day (CT = circadian time where hour 0 is defined as subjective dawn). (B) Similar to the protein, frq message undergoes molecular rhythms in abundance. mRNA levels were measured both by densitometric analysis of Northern blots and quantitative real-time PCR and show similar phase of maximum expression slightly after subjective dawn (CT0-4). This rhythmic production of mRNA is driven by the WCC. (C) Schematic of the primary structure of FRQ showing the position of the two translational start sites and domain structure and time-of-day-specific phosphorylation of FRQ as revealed by tandem mass spectroscopy. CC – coiled-coiled domain, NLS – nuclear localization signal, FCD – FRQ-CKI interacting domain, FFD – FRQ-FRH interacting domain. Domains in green provide protein-protein interaction giving FRQ a scaffold-like function and domains in red regulates stability. Phosphorylation is seen nearly over the entire protein however, here spatial and temporal domains that change phosphorylation state in concert are indicated by the blue/yellow colored gradients on the right. Degrees of phosphorylation are color coded such that the highest intensity yellow represents the time-of-day peak in phosphorylation for the corresponding region of the protein. This panel of the figure is modified from Baker et al. (2009).
Figure 4. A time line of biochemical and molecular processes in the Neurospora clock
In the late night at the left hand side, the WCC is rapidly cycling through the frq promoter (black arrows), actively driving transcription of frq and being rendered unstable in the process so that it rapidly turns over. WCC in the figure denotes the active complex only. FRQ is translated, dimerizes, and makes a stable complex with FRH. This complex interacts with the WCC and serves as a platform to introduce kinases, especially CK1 which stably associates with the complex; CK2 and other kinases are transiently associated. By mid to late subjective day CK1 and the other kinases are phosphorylating the WCC to inactivate it, and FRQ to change its structure. By the early subjective night WCC is inactivated, frq transcription is nearly stopped, and FRQ is becoming highly phosphorylated, so that by the late subjective night it transiently interacts with its ubiquitin ligase FWD-1 leading to turnover in the proteasome. After FRQ turns over in the late night, the frq promoter can be reactivate by newly synthesized WCC or by WCC reactivated through the action of protein phosphatases such as PP2A.
Similar articles
- Dissecting the mechanisms of the clock in Neurospora.
Hurley J, Loros JJ, Dunlap JC. Hurley J, et al. Methods Enzymol. 2015;551:29-52. doi: 10.1016/bs.mie.2014.10.009. Epub 2014 Dec 26. Methods Enzymol. 2015. PMID: 25662450 Free PMC article. Review. - Interlocked feedback loops of the circadian clock of Neurospora crassa.
Brunner M, Káldi K. Brunner M, et al. Mol Microbiol. 2008 Apr;68(2):255-62. doi: 10.1111/j.1365-2958.2008.06148.x. Epub 2008 Feb 26. Mol Microbiol. 2008. PMID: 18312266 Review. - Methods to study molecular mechanisms of the Neurospora circadian clock.
Cha J, Zhou M, Liu Y. Cha J, et al. Methods Enzymol. 2015;551:137-51. doi: 10.1016/bs.mie.2014.10.002. Epub 2014 Dec 26. Methods Enzymol. 2015. PMID: 25662455 Free PMC article. - Mechanism of the Neurospora circadian clock, a FREQUENCY-centric view.
Cha J, Zhou M, Liu Y. Cha J, et al. Biochemistry. 2015 Jan 20;54(2):150-6. doi: 10.1021/bi5005624. Epub 2014 Dec 30. Biochemistry. 2015. PMID: 25302868 Free PMC article. Review. - Around the Fungal Clock: Recent Advances in the Molecular Study of Circadian Clocks in Neurospora and Other Fungi.
Montenegro-Montero A, Canessa P, Larrondo LF. Montenegro-Montero A, et al. Adv Genet. 2015;92:107-84. doi: 10.1016/bs.adgen.2015.09.003. Epub 2015 Oct 27. Adv Genet. 2015. PMID: 26639917 Review.
Cited by
- Post-Translational Mechanisms of Plant Circadian Regulation.
Yan J, Kim YJ, Somers DE. Yan J, et al. Genes (Basel). 2021 Feb 24;12(3):325. doi: 10.3390/genes12030325. Genes (Basel). 2021. PMID: 33668215 Free PMC article. Review. - High-resolution spatiotemporal analysis of gene expression in real time: in vivo analysis of circadian rhythms in Neurospora crassa using a FREQUENCY-luciferase translational reporter.
Larrondo LF, Loros JJ, Dunlap JC. Larrondo LF, et al. Fungal Genet Biol. 2012 Sep;49(9):681-3. doi: 10.1016/j.fgb.2012.06.001. Epub 2012 Jun 10. Fungal Genet Biol. 2012. PMID: 22695169 Free PMC article. - Developing a Temperature-Inducible Transcriptional Rheostat in Neurospora crassa.
Tabilo-Agurto C, Del Rio-Pinilla V, Eltit-Villarroel V, Goity A, Muñoz-Guzmán F, Larrondo LF. Tabilo-Agurto C, et al. mBio. 2023 Feb 28;14(1):e0329122. doi: 10.1128/mbio.03291-22. Epub 2023 Feb 6. mBio. 2023. PMID: 36744948 Free PMC article. - The contribution of the White Collar complex to Cryptococcus neoformans virulence is independent of its light-sensing capabilities.
Zhu P, Idnurm A. Zhu P, et al. Fungal Genet Biol. 2018 Dec;121:56-64. doi: 10.1016/j.fgb.2018.09.008. Epub 2018 Sep 25. Fungal Genet Biol. 2018. PMID: 30266690 Free PMC article. - Differential regulation of phosphorylation, structure, and stability of circadian clock protein FRQ isoforms.
Chen X, Liu X, Gan X, Li S, Ma H, Zhang L, Wang P, Li Y, Huang T, Yang X, Fang L, Liang Y, Wu J, Chen T, Zhou Z, Liu X, Guo J. Chen X, et al. J Biol Chem. 2023 Apr;299(4):104597. doi: 10.1016/j.jbc.2023.104597. Epub 2023 Mar 9. J Biol Chem. 2023. PMID: 36898580 Free PMC article.
References
- Allada R, White NE, So WV, Hall JC, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. - PubMed
- Aronson BD, Johnson KA, Loros JJ, Dunlap JC. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science. 1994;263:1578–1584. - PubMed
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. - PubMed
- Ballario P, Talora C, Galli D, Linden H, Macino G. Roles in dimerization and blue light photoresponse of the PAS and LOV domains of Neurospora crassa white collar proteins. Mol Microbiol. 1998;29:719–729. - PubMed
Publication types
MeSH terms
Grants and funding
- R37 GM034985/GM/NIGMS NIH HHS/United States
- P01 GM068087-09/GM/NIGMS NIH HHS/United States
- 5T32GM008704/GM/NIGMS NIH HHS/United States
- R01 GM083336-22/GM/NIGMS NIH HHS/United States
- T32 GM008704/GM/NIGMS NIH HHS/United States
- GM083336/GM/NIGMS NIH HHS/United States
- GM68087/GM/NIGMS NIH HHS/United States
- R01 GM083336/GM/NIGMS NIH HHS/United States
- R01 GM034985/GM/NIGMS NIH HHS/United States
- R01 GM034985-26/GM/NIGMS NIH HHS/United States
- P01 GM068087/GM/NIGMS NIH HHS/United States
- GM34985/GM/NIGMS NIH HHS/United States