Electrostatic interactions involving the extreme C terminus of nuclear export factor CRM1 modulate its affinity for cargo - PubMed (original) (raw)
Electrostatic interactions involving the extreme C terminus of nuclear export factor CRM1 modulate its affinity for cargo
Abigail M Fox et al. J Biol Chem. 2011.
Abstract
The toroid-shaped nuclear protein export factor CRM1 is constructed from 21 tandem HEAT repeats, each of which contains an inner (B) and outer (A) α-helix joined by loops. Proteins targeted for export have a nuclear export signal (NES) that binds between the A-helices of HEAT repeats 11 and 12 on the outer surface of CRM1. RanGTP binding increases the affinity of CRM1 for NESs. In the absence of RanGTP, the CRM1 C-terminal helix, together with the HEAT repeat 9 loop, modulates its affinity for NESs. Here we show that there is an electrostatic interaction between acidic residues at the extreme distal tip of the C-terminal helix and basic residues on the HEAT repeat 12 B-helix that lies on the inner surface of CRM1 beneath the NES binding site. Small angle x-ray scattering indicates that the increased affinity for NESs generated by mutations in the C-terminal helix is not associated with large scale changes in CRM1 conformation, consistent with the modulation of NES affinity being mediated by a local change in CRM1 near the NES binding site. These data also suggest that in the absence of RanGTP, the C-terminal helix lies across the CRM1 toroid in a position similar to that seen in the CRM1-Snurportin crystal structure. By creating local changes that stabilize the NES binding site in its closed conformation and thereby reducing the affinity of CRM1 for NESs, the C-terminal helix and HEAT 9 loop facilitate release of NES-containing cargo in the cytoplasm and also inhibit their return to the nucleus.
Figures
FIGURE 1.
Alignment of CRM1 C-terminal helix sequences. The secondary structure of the human CRM1 chain from the CRM1-Snurportin complex (9) is shown above the sequences. The last residue for which there is clear electron density in the crystal structures is Pro-1061. Acidic and basic residues of the C-terminal helices are shown in red and blue, respectively. Note the conservation of acidic residues at the extreme C terminus.
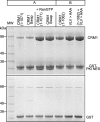
FIGURE 2.
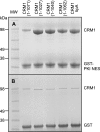
Binding of CRM1 C-terminal helix variants to immobilized GST-PKI NES. A, whereas wild type CRM1 bound weakly to GST-PKI NES beads in pull-down experiments, all of the variant CRM1 constructs showed much stronger binding, consistent with the acidic residues at the extreme C terminus being critical. B shows the same proteins bound to the GST alone control resin.
FIGURE 3.
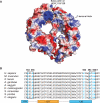
The conserved basic patch on CRM1. A, electrostatic surface potential of human CRM1 from the CRM1-Snurportin crystal structure (9). Regions of acidic and basic potential are shown in red and blue, respectively. The basic patch on HEAT repeats 11B and 12B lies adjacent to the end of the C-terminal helix of CRM1. B, the HEAT 11B-12B regions of several species of CRM1 are aligned, showing the conservation of the six basic patch residues (in blue). HEAT repeats are indicated below the alignment.
FIGURE 4.
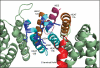
Conserved basic residues on HEAT 11B/12B of CRM1. The conserved basic residues (Arg-553, Arg-556, Lys-590, Lys-594, Arg-596, and Arg-597) shown in dark blue lie at the end of the HEAT 11B/12B helices closest to the end of the C-terminal helix (as modeled by Dong et al. (9)). The NES binds on the opposite side of the CRM1 backbone at the A helices of these HEAT repeats.
FIGURE 5.
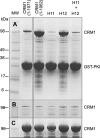
Binding of CRM1 basic patch variants to immobilized GST-PKI NES. A, pulldowns were carried out using cell lysates incubated with resin that had been preloaded with GST-PKI NES. B, none of the variants bound to GST alone. C, lysate samples incubated with Ni-NTA show that all of the lysates contained comparable amounts of CRM1. Variant H11 was R553A/R556A, whereas variant H12 was K594A/R596A/R597A.
FIGURE 6.
Binding of the Swap (A) and HEAT 9 loop (B) CRM1 variants to immobilized GST-PKI NES. Both wild type CRM1 (1–1071) and the CRM1-Swap variant bound to GST-PKI NES with a lower affinity than the tip deletion variant (1–1062). The addition of RanGTP significantly increased the affinity of wild type and CRM1-Swap for the NES. The HEAT 9 loop variant binds the NES with a higher affinity than wild type CRM1 in the absence of RanGTP, and the binding is comparable with that seen for the variant that combines the C-terminal helix tip deletion and HEAT 9 loop mutation. The variants all bound to the GST control with similar affinity (lower panel).
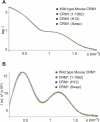
FIGURE 7.
SAXS analysis of CRM1 and variants. A, Kratky plot of experimental mouse CRM1 SAXS data overlaid with simulated plots derived from crystal structures of human CRM1 (9), mouse CRM1 (10), and S. cerevisiae Xpo1p (11). B, simulated plots as in A overlaid with experimental S. cerevisiae Xpo1p SAXS data. C, effect on the Kratky plot of human CRM1 (9) of removing the density of the extended C-terminal helix from the crystal structure. D, χ2 values for the comparisons shown in the Kratky plots (A–C). The best fit was obtained between the mouse CRM1 experimental SAXS data and the structure of human CRM1 described by Dong et al. (9). Removing the C-terminal helix from this structure, in which it lies across the toroid, increases χ2 from 5 to 14.2.
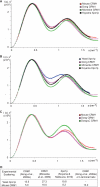
FIGURE 8.
SAXS data for wild type CRM1 and C-terminal helix and basic patch variants. Shown are overlaid SAXS curves (A) and Kratky plots (B) of the three variants (CRM1-1–1062, CRM1 H12, and CRM1-Swap) and wild type CRM1. None of the mutations produced a significant change in the SAXS pattern, indicating that they did not generate a major conformational change in CRM1.
FIGURE 9.
The HEAT 11/12 B helices can accommodate binding of both the C-terminal helix tip and the HEAT 9 loop. Alignment of S. cerevisiae Xpo1p (11) and human CRM1 (9) indicates that the two proposed interactions at the HEAT 11/12 B helices (in cyan) cleft could occur simultaneously. The HEAT 9 loop is shown in the conformation that promotes closure of the NES cleft at the HEAT 11/12 A helices.
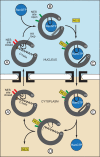
FIGURE 10.
Model for the function of the C-terminal helix and the HEAT 9 loop in modulating CRM1 cargo binding and release during the nuclear protein export cycle. The unbound state of CRM1 has a low affinity for the NES because of the combined autoinhibitory effect of the HEAT 9 loop and the C-terminal helix (A). In the nucleus, RanGTP binding sequesters the HEAT 9 loop and displaces the C-terminal helix (B), generating a high affinity state of CRM1 in which the open state of the NES binding site is favored. This, in turn, leads to NES binding and the formation of a ternary export complex (C). The ternary complex diffuses through nuclear pore complexes to the cytoplasm, where it is disassembled. RanGTP is removed by the action of RanGAP, RanBP1, and nucleoporins. In the absence of RanGTP (D), the HEAT 9 loop and the C-terminal helix can return to their autoinhibitory positions at the HEAT 11/12 B helices, facilitating NES release and the reversion of CRM1 to the low affinity state that then diffuses back to the nucleus where it can participate in a further export cycle. The proposed C-terminal tip electrostatic interaction is shown as a brown ellipse. HEAT repeats 11 and 12 of the NES binding site are modeled in red to indicate a closed state or green to indicate an open state. Dashed lines indicate regions in the Dong et al. (9) crystal structure, for which there was insufficient electron density to model the chain.
Similar articles
- An allosteric mechanism to displace nuclear export cargo from CRM1 and RanGTP by RanBP1.
Koyama M, Matsuura Y. Koyama M, et al. EMBO J. 2010 Jun 16;29(12):2002-13. doi: 10.1038/emboj.2010.89. Epub 2010 May 18. EMBO J. 2010. PMID: 20485264 Free PMC article. - A 2.1-Å-resolution crystal structure of unliganded CRM1 reveals the mechanism of autoinhibition.
Saito N, Matsuura Y. Saito N, et al. J Mol Biol. 2013 Jan 23;425(2):350-64. doi: 10.1016/j.jmb.2012.11.014. Epub 2012 Nov 16. J Mol Biol. 2013. PMID: 23164569 - A cellular reporter to evaluate CRM1 nuclear export activity: functional analysis of the cancer-related mutant E571K.
García-Santisteban I, Arregi I, Alonso-Mariño M, Urbaneja MA, Garcia-Vallejo JJ, Bañuelos S, Rodríguez JA. García-Santisteban I, et al. Cell Mol Life Sci. 2016 Dec;73(24):4685-4699. doi: 10.1007/s00018-016-2292-0. Epub 2016 Jun 16. Cell Mol Life Sci. 2016. PMID: 27312238 Free PMC article. - Allosteric control of the exportin CRM1 unraveled by crystal structure analysis.
Monecke T, Dickmanns A, Ficner R. Monecke T, et al. FEBS J. 2014 Sep;281(18):4179-94. doi: 10.1111/febs.12842. Epub 2014 Jun 6. FEBS J. 2014. PMID: 24823279 Free PMC article. Review. - Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents.
Turner JG, Dawson J, Cubitt CL, Baz R, Sullivan DM. Turner JG, et al. Semin Cancer Biol. 2014 Aug;27:62-73. doi: 10.1016/j.semcancer.2014.03.001. Epub 2014 Mar 12. Semin Cancer Biol. 2014. PMID: 24631834 Free PMC article. Review.
Cited by
- Novel-and Not So Novel-Inhibitors of the Multifunctional CRM1 Protein.
Aumann WK, Kazi R, Harrington AM, Wechsler DS. Aumann WK, et al. Oncol Rev. 2024 Aug 5;18:1427497. doi: 10.3389/or.2024.1427497. eCollection 2024. Oncol Rev. 2024. PMID: 39161560 Free PMC article. Review. - The Role of Protein Disorder in Nuclear Transport and in Its Subversion by Viruses.
Wubben JM, Atkinson SC, Borg NA. Wubben JM, et al. Cells. 2020 Dec 10;9(12):2654. doi: 10.3390/cells9122654. Cells. 2020. PMID: 33321790 Free PMC article. Review. - Karyopherin Kap114p-mediated trans-repression controls ribosomal gene expression under saline stress.
Liao CC, Shankar S, Pi WC, Chang CC, Ahmed GR, Chen WY, Hsia KC. Liao CC, et al. EMBO Rep. 2020 Jul 3;21(7):e48324. doi: 10.15252/embr.201948324. Epub 2020 Jun 2. EMBO Rep. 2020. PMID: 32484313 Free PMC article. - Mechanistic insights from the recent structures of the CRM1 nuclear export complex and its disassembly intermediate.
Koyama M, Matsuura Y. Koyama M, et al. Biophysics (Nagoya-shi). 2012 Nov 30;8:145-50. doi: 10.2142/biophysics.8.145. eCollection 2012. Biophysics (Nagoya-shi). 2012. PMID: 27493531 Free PMC article. Review. - Enzymatically driven transport: a kinetic theory for nuclear export.
Kim S, Elbaum M. Kim S, et al. Biophys J. 2013 Nov 5;105(9):1997-2005. doi: 10.1016/j.bpj.2013.09.011. Biophys J. 2013. PMID: 24209844 Free PMC article.
References
- Fornerod M., Ohno M., Yoshida M., Mattaj I. W. (1997) Cell 90, 1051–1060 - PubMed
- Stade K., Ford C. S., Guthrie C., Weis K. (1997) Cell 90, 1041–1050 - PubMed
- Weis K. (2003) Cell 112, 441–451 - PubMed
- Kutay U., Güttinger S. (2005) Trends Cell Biol. 15, 121–124 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases