Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes - PubMed (original) (raw)
. 2011 Jul 12;108(28):11578-83.
doi: 10.1073/pnas.1010678108. Epub 2011 Jun 27.
Justin W Heizer, Yuan Li, Kathryn Chapman, Carol Anne Ogden, Karl Andreasen, Ellen Shapland, Gary Kucera, Jennifer Mogan, Jessica Humann, Laurel L Lenz, Alastair D Morrison, Anne-Laure Perraud
Affiliations
- PMID: 21709234
- PMCID: PMC3136283
- DOI: 10.1073/pnas.1010678108
Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes
Heather Knowles et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The generation of reactive oxygen species (ROS) is inherent to immune responses. ROS are crucially involved in host defense against pathogens by promoting bacterial killing, but also as signaling agents coordinating the production of cytokines. Transient Receptor Potential Melastatin 2 (TRPM2) is a Ca(2+)-permeable channel gated via binding of ADP-ribose, a metabolite formed under conditions of cellular exposure to ROS. Here, we show that TRPM2-deficient mice are extremely susceptible to infection with Listeria monocytogenes (Lm), exhibiting an inefficient innate immune response. In a comparison with IFNγR-deficient mice, TRPM2(-/-) mice shared similar features of uncontrolled bacterial replication and reduced levels of inducible (i)NOS-expressing monocytes, but had intact IFNγ responsiveness. In contrast, we found that levels of cytokines IL-12 and IFNγ were diminished in TRPM2(-/-) mice following Lm infection, which correlated with their reduced innate activation. Moreover, TRPM2(-/-) mice displayed a higher degree of susceptibility than IL-12-unresponsive mice, and supplementation with recombinant IFNγ was sufficient to reverse the unrestrained bacterial growth and ultimately the lethal phenotype of Lm-infected TRPM2(-/-) mice. The severity of listeriosis we observed in TRPM2(-/-) mice has not been reported for any other ion channel. These findings establish an unsuspected role for ADP-ribose and ROS-mediated cation flux for innate immunity, opening up unique possibilities for immunomodulatory intervention through TRPM2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
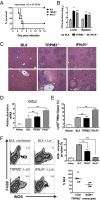
TRPM2 is required for survival and control of bacterial burden in L. monocytogenes infection. (A) TRPM2−/− mice are as susceptible to Lm infection as IFNγR−/− mice. Kaplan–Meier survival plot of mice i.v. infected with Lm. Eight BL6 (squares), TRPM2−/− (circles), or IFNγR−/− mice (triangles) were infected with 5.5 × 103 Lm. Median survival of TRPM2−/− mice closely matched IFNγR−/− mice and both are significant by log rank test (P < 0.005) compared with BL6. Data are representative of two independent experiments. (_B_) _Lm_ burden in liver and spleen during early infection. BL6, TRPM2−/−, and IFNγR−/− mice (three to five mice per group) infected with 2 × 103 _Lm_ and cfu determined 48 hpi. Mean value and SEM are indicated (**_P_ < 0.01, ***_P_ < 0.001). Data are representative of five experiments. (_C_) Comparison of liver histopathology of _Lm_ infected mice. Representative parenchyma (_Upper_) and perivascular (_Lower_) images from H&E stained BL6 livers infected with 5 × 103 _Lm_ compared with TRPM2−/− and IFNγR−/− moribund mice from _A_, day 4. Original magnification 200×; dotted lines outline areas of necrosis; asterisks denote blood vessel. (_D_) _cxcl2_ expression in response to _Lm_ infection. Collagenase digested splenocytes from naïve BL6 (_n_ = 3), naïve TRPM2−/− (_n_ = 2), or mice infected for 24 h with 4.5 x 103 _Lm_ (_n_ = 4 of each genotype) and _cxcl2_ mRNA quantified using qPCR. All naïve mice (BL6 and TRPM2−/−) had equivalent _cxcl2_ expression and were averaged together. Bars represent means ± SEM. NS indicates _P_ > 0.05, **P < 0.01. (E) Average frequency of Ly6Ghi gated neutrophils per spleen from naïve or mice infected as in B. Bars represent mean ± SEM, four mice per group and five independent experiments. (F) In vivo activation of iNOS by Ly6Glow Ly6C+ monocytes is impaired in response to Lm infection in TRPM2−/− mice. BL6, TRPM2−/−, and IFNγR−/− (n = 4 each) mice infected as in B. Representative density plots of iNOS expression in CD11b+/Ly6Glow/Ly6C+ gated monocytes. Average frequency of iNOS+ monocytes is significantly reduced in TRPM2−/−- and IFNγR−/−-infected mice. Bars represent mean frequency ± SEM **P < 0.01, ***P < 0.001. (Lower) Comparison of the mean absolute number of total or iNOS+ TRPM2−/− to total or iNOS+ BL6 monocytes at 48 hpi from five separate experiments (# TRPM2−/− cells ÷ # BL6 cells × 100).
Fig. 2.
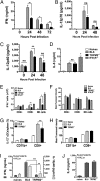
TRPM2−/− mice have diminished _L. monocytogenes_-induced cytokine production. TRPM2−/− mice exhibit reductions in serum cytokines IFNγ (A), IL-12p70 (B), and IL-12p40 (C) in response to Lm infection. BL6 and TRPM2−/− mice were infected with 5 × 103 Lm for the times indicated and serum assayed by ELISA. Bars represent mean ± SEM from three to five mice per group per time point. Data are representative of three independent experiments at 24 hpi, five experiments at 48 hpi, and two experiments at 72 hpi. (D) TRPM2−/− mice have normal serum IL-6 production in response to Lm infection. BL6, TRPM2−/−, and IFNγR−/− mice were infected with 8 × 103 Lm and serum IL-6 measured by ELISA at 24 hpi. Bars represent mean ± SEM from three to five mice per group and data are representative of two independent experiments. (E) TRPM2−/− mice have reduced numbers of IFNγ+ cells in response to Lm infection. BL6, TRPM2−/−, and IFNγR−/− mice infected with 8 × 103 Lm for 24 hpi and absolute numbers of gated NK1.1neg CD3+ T lymphocytes and NK1.1+ CD3neg NK cells expressing IFNγ are shown (
Fig. S3
shows example of dot blots). Bars represent mean ± SEM for three to five mice per group. Data are representative of three independent experiments comparing BL6 with TRPM2−/− mice and two additional experiments including IFNγR−/− mice. (F) Mean fluorescent intensity (MFI) of IFNγ-gated cells is similarly increased in response to infection. Bars represent MFI ± SEM for IFNγ+-gated cells in E. (G) TRPM2−/− mice have a reduced frequency of IL-12+ CD8+ CD11chigh (cDCs) in response to Lm infection. BL6 and TRPM2−/− mice infected with 6 × 103 Lm for 24 hpi and frequency of gated CD11chigh CD8+ cDCs expressing IL-12p40 are shown. (
Fig. S3
shows representative dot blots). Bars represent mean ± SEM for four to five mice per group. Data are representative of two independent experiments comparing BL6 with TRPM2−/− mice at 24 hpi and one experiment at 48 hpi. (H) MFI of IL-12p40–gated cells is similarly increased in response to infection. Bars represent MFI ± SEM for IL-12p40–gated cells in G. (I) Lm_-infected TRPM2−/− splenocytes have impaired IFNγ and IL-12p40 secretion in vitro. Collagenase digested splenocytes from BL6 and TRPM2−/− mice (pooled naïves, n = 2 of each genotype) or infected (n = 4, each) with 4.5 × 103 Lm for 24 h were cultured in vitro for 48 h with 108/mL HK_Lm and supernatants assayed for secreted cytokines by ELISA. Bars represent mean ± SEM and are representative of three separate experiments. (J) _Lm_-infected TRPM2−/− splenocytes secrete normal amounts of IL-6 in vitro. Collagenase digested splenocytes from BL6 and TRPM2−/− mice (pooled naïves, n = 3 each) or infected (n = 3–5, each) with 3 × 103 Lm for 48 h were cultured as in G. Bars represent mean ± SEM and are representative of three separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 3.
IL-12 is indispensable for low-dose infection of TRPM2−/− mice. (A) TRPM2−/− mice can survive a lower dose Lm infection. Kaplan–Meier survival plot of 10 BL6 (squares), 10 TRPM2−/− (circles), or 6 IFNγR−/− mice (triangles) infected i.v. with 2 × 103 Lm. Median survival of IFNγR−/− mice (4.75 d) was significantly reduced by log rank test (P < 0.0001) compared with BL6 and TRPM2−/− mice. Data representative of four separate experiments. (B) TRPM2−/− mice are susceptible to a low-dose Lm infection where IL-12Rβ2−/− mice are resistant. Kaplan–Meier survival plot of eight BL6 (squares), four TRPM2−/− (open circles), or four IL-12Rβ2−/− mice (open triangles), or five IFNγR−/− mice (closed triangles) infected with 3 × 103 Lm. Median survival of TRPM2−/− mice closely matched IFNγR−/− mice (3.5 d) and both are significant by log rank test (P < 0.0001) compared with BL6 and IL-12Rβ2−/− mice. Data are representative of two separate experiments. (C) IL-12p40 is indispensable in TRPM2−/− mice infected with very low doses of Lm. Kaplan–Meier survival plot of BL6 (squares), TRPM2−/− (circles), or IFNγR−/− mice (triangles) infected i.v. with 1 × 103 Lm (five to eight mice each group). Three hours before infection, BL6 or TRPM2−/− mice were i.p. injected with 1.5 mg neutralizing mAb against IL-12p40 (white) or rat isotype control IgG2A (black). Median survival of IFNγR−/− mice (7.75 d) was significant by log rank test (P < 0.005) compared with BL6 and TRPM2−/−. Data are representative of two separate experiments.
Fig. 4.
Recombinant IFNγ treatment is sufficient to reverse TRPM2−/− susceptibility to high-dose Lm infection. (A and B) Pretreating mice with rIFNγ improves Lm burden in liver and spleen. BL6 and TRPM2−/− mice were i.v. injected with either recombinant mouse IFNγ (105 U/each) in PBS or control PBS and 18 h later, infected i.v. with 6 × 103 Lm. Colony-forming units in both liver and spleen were determined 48 hpi (A) and 72 hpi (B). Mean value and SEM are indicated (NS indicates P > 0.05, *P < 0.05). Data are representative of three experiments. (C) Pretreating mice with rIFNγ reverses serum ALT elevation in _Lm_-infected TRPM2−/− mice. Serum was obtained from BL6 and TRPM2−/− mice treated as in A and B (n = 5 each group) and ALT quantified using a modified Reitman Frankel colorimetric endpoint reaction. Floating bars represent range, and the line is the mean. TRPM2−/− mice receiving rIFNγ had a significant reversal of ALT, **P < 0.01. (D) Recombinant IFNγ treatment is sufficient to reverse mortality of TRPM2−/− mice to Lm infection. Kaplan–Meier survival plot of mice i.v. infected with Lm. BL6 (squares) and TRPM2−/− (circles) were i.v. injected with mouse rIFNγ (105 U/each) in PBS (white), or control PBS (black) and 18 h later, infected i.v. with 4 × 103 Lm. Median survival of rIFNγ-treated TRPM2−/− mice was significantly improved from untreated TRPM2−/− mice (3.5 d) by log rank test (P < 0.05).
Similar articles
- The TRPM2 ion channel contributes to cytokine hyperproduction in a mouse model of Down Syndrome.
Gally F, Rao DM, Schmitz C, Colvin KL, Yeager ME, Perraud AL. Gally F, et al. Biochim Biophys Acta Mol Basis Dis. 2018 Jan;1864(1):126-132. doi: 10.1016/j.bbadis.2017.09.025. Epub 2017 Sep 30. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28970008 - The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation.
Knowles H, Li Y, Perraud AL. Knowles H, et al. Immunol Res. 2013 Mar;55(1-3):241-8. doi: 10.1007/s12026-012-8373-8. Immunol Res. 2013. PMID: 22975787 Review. - Expression of the p60 autolysin enhances NK cell activation and is required for listeria monocytogenes expansion in IFN-gamma-responsive mice.
Humann J, Bjordahl R, Andreasen K, Lenz LL. Humann J, et al. J Immunol. 2007 Feb 15;178(4):2407-14. doi: 10.4049/jimmunol.178.4.2407. J Immunol. 2007. PMID: 17277147 - Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice.
Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Seki E, et al. J Immunol. 2002 Oct 1;169(7):3863-8. doi: 10.4049/jimmunol.169.7.3863. J Immunol. 2002. PMID: 12244183 - TRPM2: a multifunctional ion channel for calcium signalling.
Sumoza-Toledo A, Penner R. Sumoza-Toledo A, et al. J Physiol. 2011 Apr 1;589(Pt 7):1515-25. doi: 10.1113/jphysiol.2010.201855. Epub 2010 Dec 6. J Physiol. 2011. PMID: 21135052 Free PMC article. Review.
Cited by
- TRPM2 facilitates tumor progression of clear cell renal cell carcinoma by relieving Endoplasmic Reticulum Stress.
Yuan H, Li W, Lou N, Yu T, Meng X, Xiao W, Zhang X. Yuan H, et al. Int J Med Sci. 2023 Jan 1;20(1):57-69. doi: 10.7150/ijms.77944. eCollection 2023. Int J Med Sci. 2023. PMID: 36619219 Free PMC article. - DAMP-sensing receptors in sterile inflammation and inflammatory diseases.
Gong T, Liu L, Jiang W, Zhou R. Gong T, et al. Nat Rev Immunol. 2020 Feb;20(2):95-112. doi: 10.1038/s41577-019-0215-7. Epub 2019 Sep 26. Nat Rev Immunol. 2020. PMID: 31558839 Review. - Structures and gating mechanism of human TRPM2.
Wang L, Fu TM, Zhou Y, Xia S, Greka A, Wu H. Wang L, et al. Science. 2018 Dec 21;362(6421):eaav4809. doi: 10.1126/science.aav4809. Epub 2018 Nov 22. Science. 2018. PMID: 30467180 Free PMC article. - Ion channelopathies of the immune system.
Vaeth M, Feske S. Vaeth M, et al. Curr Opin Immunol. 2018 Jun;52:39-50. doi: 10.1016/j.coi.2018.03.021. Epub 2018 Apr 7. Curr Opin Immunol. 2018. PMID: 29635109 Free PMC article. Review. - CD38 controls the innate immune response against Listeria monocytogenes.
Lischke T, Heesch K, Schumacher V, Schneider M, Haag F, Koch-Nolte F, Mittrücker HW. Lischke T, et al. Infect Immun. 2013 Nov;81(11):4091-9. doi: 10.1128/IAI.00340-13. Epub 2013 Aug 26. Infect Immun. 2013. PMID: 23980105 Free PMC article.
References
- Temkin V, Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: The receptor-interacting protein 1 odyssey. Immunol Rev. 2007;220:8–21. - PubMed
- Perraud AL, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI065638/AI/NIAID NIH HHS/United States
- R01 AI065638/AI/NIAID NIH HHS/United States
- R01 AI065638-03/AI/NIAID NIH HHS/United States
- R56 AI065638/AI/NIAID NIH HHS/United States
- R21 AI055701/AI/NIAID NIH HHS/United States
- AI055701/AI/NIAID NIH HHS/United States
- T32 AI007405/AI/NIAID NIH HHS/United States
- R01 AI055701/AI/NIAID NIH HHS/United States
- 5 T32 AI07405/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous