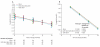Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2011 Jul 23;378(9788):319-27.
doi: 10.1016/S0140-6736(11)60895-7. Epub 2011 Jun 27.
Brian Bundy, Dorothy J Becker, Linda A DiMeglio, Stephen E Gitelman, Robin Goland, Peter A Gottlieb, Carla J Greenbaum, Kevan C Herold, Jennifer B Marks, Roshanak Monzavi, Antoinette Moran, Tihamer Orban, Jerry P Palmer, Philip Raskin, Henry Rodriguez, Desmond Schatz, Darrell M Wilson, Jeffrey P Krischer, Jay S Skyler; Type 1 Diabetes TrialNet GAD Study Group
Collaborators, Affiliations
- PMID: 21714999
- PMCID: PMC3580128
- DOI: 10.1016/S0140-6736(11)60895-7
Randomized Controlled Trial
Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial
Diane K Wherrett et al. Lancet. 2011.
Abstract
Background: Glutamic acid decarboxylase (GAD) is a major target of the autoimmune response that occurs in type 1 diabetes mellitus. In animal models of autoimmunity, treatment with a target antigen can modulate aggressive autoimmunity. We aimed to assess whether immunisation with GAD formulated with aluminum hydroxide (GAD-alum) would preserve insulin production in recent-onset type 1 diabetes.
Methods: Patients aged 3-45 years who had been diagnosed with type 1 diabetes for less than 100 days were enrolled from 15 sites in the USA and Canada, and randomly assigned to receive one of three treatments: three injections of 20 μg GAD-alum, two injections of 20 μg GAD-alum and one of alum, or 3 injections of alum. Injections were given subcutaneously at baseline, 4 weeks later, and 8 weeks after the second injection. The randomisation sequence was computer generated at the TrialNet coordinating centre. Patients and study personnel were masked to treatment assignment. The primary outcome was the baseline-adjusted geometric mean area under the curve (AUC) of serum C-peptide during the first 2 h of a 4-h mixed meal tolerance test at 1 year. Secondary outcomes included changes in glycated haemoglobin A(1c) (HbA(1c)) and insulin dose, and safety. Analysis included all randomised patients with known measurements. This trial is registered with ClinicalTrials.gov, number NCT00529399.
Findings: 145 patients were enrolled and treated with GAD-alum (n=48), GAD-alum plus alum (n=49), or alum (n=48). At 1 year, the 2-h AUC of C-peptide, adjusted for age, sex, and baseline C-peptide value, was 0·412 nmol/L (95% CI 0·349-0·478) in the GAD-alum group, 0·382 nmol/L (0·322-0·446) in the GAD-alum plus alum group, and 0·413 nmol/L (0·351-0·477) in the alum group. The ratio of the population mean of the adjusted geometric mean 2-h AUC of C-peptide was 0·998 (95% CI 0·779-1·22; p=0·98) for GAD-alum versus alum, and 0·926 (0·720-1·13; p=0·50) for GAD-alum plus alum versus alum. HbA(1c), insulin use, and the occurrence and severity of adverse events did not differ between groups.
Interpretation: Antigen-based immunotherapy therapy with two or three doses of subcutaneous GAD-alum across 4-12 weeks does not alter the course of loss of insulin secretion during 1 year in patients with recently diagnosed type 1 diabetes. Although antigen-based therapy is a highly desirable treatment and is effective in animal models, translation to human autoimmune disease remains a challenge.
Funding: US National Institutes of Health.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Figure 1
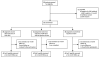
(A): Enrollment, Randomization, and Follow-up of Study Participants. (B): Rate of enrollment over time. Indicated is the point at which approval was granted to change the lower age for eligibility of enrollment from 16 years to 3 years.
Figure 1
(A): Enrollment, Randomization, and Follow-up of Study Participants. (B): Rate of enrollment over time. Indicated is the point at which approval was granted to change the lower age for eligibility of enrollment from 16 years to 3 years.
Figure 2
(A): The population mean (and 95% confidence intervals) of stimulated C-peptide 2-hour AUC mean (expressed as nmol/L) over time for each treatment group. The estimates are from the analysis of covariance model adjusting for age, gender, baseline value of C-peptide, and treatment assignment. Y-axis is on a log(y + 1) scale. (B): The fitted line representing the population mean of stimulated C-peptide 2-hour AUC mean over time for each treatment group. The estimates are from the analysis of mixed effects model adjusting for age, gender, baseline value of C-peptide, and treatment assignment, and including a fixed effect for time as a linear line on the log(y + 1) scale. (C): The proportion of subjects with 2 hour peak C-peptide remaining at or above 0.2 nmol/L over time for each treatment group.
Figure 2
(A): The population mean (and 95% confidence intervals) of stimulated C-peptide 2-hour AUC mean (expressed as nmol/L) over time for each treatment group. The estimates are from the analysis of covariance model adjusting for age, gender, baseline value of C-peptide, and treatment assignment. Y-axis is on a log(y + 1) scale. (B): The fitted line representing the population mean of stimulated C-peptide 2-hour AUC mean over time for each treatment group. The estimates are from the analysis of mixed effects model adjusting for age, gender, baseline value of C-peptide, and treatment assignment, and including a fixed effect for time as a linear line on the log(y + 1) scale. (C): The proportion of subjects with 2 hour peak C-peptide remaining at or above 0.2 nmol/L over time for each treatment group.
Figure 3
(A): The population mean of HbA1c over time for each treatment group. The estimates are from the analysis of covariance model adjusting for age, gender, baseline value of HbA1c, and treatment assignment. (B): The population mean of insulin use per kilogram of body weight over time (in 3 month interval) for each treatment group. The estimates are from the analysis of covariance model adjusting for age, gender, baseline value of HbA1c, and treatment assignment.
Figure 4
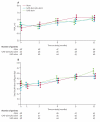
(A): The ratio (GAD-alum ×3 to alum ×3) of treatment effect on 1 year stimulated C-peptide AUC mean within categories of pre-specified baseline factors. The estimates are from the analysis of covariance modeling log of C-peptide adjusting for age, gender, baseline value of C-peptide, the indicated categorized factor, treatment assignment, and treatment interaction terms. The homogeneity test of treatment effect was significant for DR3 allele status (p = 0.04). (B): The ratio (GAD-alum ×2 to alum ×3) of treatment effect on 1 year stimulated C-peptide AUC mean within categories of pre-specified baseline factors. The estimates are from the analysis of covariance modeling log of C-peptide adjusting for age, gender, baseline value of C-peptide, the indicated categorized factor, treatment assignment, and treatment interaction terms.
Comment in
- Arresting type 1 diabetes after diagnosis: GAD is not enough.
Mathieu C, Gillard P. Mathieu C, et al. Lancet. 2011 Jul 23;378(9788):291-2. doi: 10.1016/S0140-6736(11)60978-1. Epub 2011 Jun 27. Lancet. 2011. PMID: 21715000 No abstract available.
Similar articles
- GAD treatment and insulin secretion in recent-onset type 1 diabetes.
Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R. Ludvigsson J, et al. N Engl J Med. 2008 Oct 30;359(18):1909-20. doi: 10.1056/NEJMoa0804328. Epub 2008 Oct 8. N Engl J Med. 2008. PMID: 18843118 Clinical Trial. - Safety and efficacy of autoantigen-specific therapy with 2 doses of alum-formulated glutamate decarboxylase in children with multiple islet autoantibodies and risk for type 1 diabetes: A randomized clinical trial.
Elding Larsson H, Lundgren M, Jonsdottir B, Cuthbertson D, Krischer J; DiAPREV-IT Study Group. Elding Larsson H, et al. Pediatr Diabetes. 2018 May;19(3):410-419. doi: 10.1111/pedi.12611. Epub 2017 Nov 24. Pediatr Diabetes. 2018. PMID: 29171140 Clinical Trial. - Extended evaluation of the safety and efficacy of GAD treatment of children and adolescents with recent-onset type 1 diabetes: a randomised controlled trial.
Ludvigsson J, Hjorth M, Chéramy M, Axelsson S, Pihl M, Forsander G, Nilsson NÖ, Samuelsson BO, Wood T, Aman J, Ortqvist E, Casas R. Ludvigsson J, et al. Diabetologia. 2011 Mar;54(3):634-40. doi: 10.1007/s00125-010-1988-1. Epub 2010 Nov 30. Diabetologia. 2011. PMID: 21116604 Clinical Trial. - Therapy with GAD in diabetes.
Ludvigsson J. Ludvigsson J. Diabetes Metab Res Rev. 2009 May;25(4):307-15. doi: 10.1002/dmrr.941. Diabetes Metab Res Rev. 2009. PMID: 19267332 Review. - GAD-alum immunotherapy in Type 1 diabetes mellitus.
Morales AE, Thrailkill KM. Morales AE, et al. Immunotherapy. 2011 Mar;3(3):323-32. doi: 10.2217/imt.11.9. Immunotherapy. 2011. PMID: 21395375 Review.
Cited by
- Physiological and pathogenic T cell autoreactivity converge in type 1 diabetes.
Eugster A, Lorenc A, Kotrulev M, Kamra Y, Goel M, Steinberg-Bains K, Sabbah S, Dietz S, Bonifacio E, Peakman M, Gomez-Tourino I. Eugster A, et al. Nat Commun. 2024 Oct 29;15(1):9204. doi: 10.1038/s41467-024-53255-9. Nat Commun. 2024. PMID: 39472557 Free PMC article. - Comparative Ability of Various Immunosuppressants as Adjuvants on the Activity of T1D Vaccine.
Wang X, Xie M, Li T, Shi J, Wu M, Zhang S, Sun J, Hu Y. Wang X, et al. Vaccines (Basel). 2024 Sep 29;12(10):1117. doi: 10.3390/vaccines12101117. Vaccines (Basel). 2024. PMID: 39460283 Free PMC article. - Unraveling the Potential of γ-Aminobutyric Acid: Insights into Its Biosynthesis and Biotechnological Applications.
Zhu L, Wang Z, Gao L, Chen X. Zhu L, et al. Nutrients. 2024 Aug 19;16(16):2760. doi: 10.3390/nu16162760. Nutrients. 2024. PMID: 39203897 Free PMC article. Review. - Early Metabolic Endpoints Identify Persistent Treatment Efficacy in Recent-Onset Type 1 Diabetes Immunotherapy Trials.
Jacobsen LM, Cuthbertson D, Bundy BN, Atkinson MA, Moore W, Haller MJ, Russell WE, Gitelman SE, Herold KC, Redondo MJ, Sims EK, Wherrett DK, Moran A, Pugliese A, Gottlieb PA, Sosenko JM, Ismail HM; Type 1 Diabetes TrialNet Study Group. Jacobsen LM, et al. Diabetes Care. 2024 Jun 1;47(6):1048-1055. doi: 10.2337/dc24-0171. Diabetes Care. 2024. PMID: 38621411 - Islet autoantibodies as precision diagnostic tools to characterize heterogeneity in type 1 diabetes: a systematic review.
Felton JL, Redondo MJ, Oram RA, Speake C, Long SA, Onengut-Gumuscu S, Rich SS, Monaco GSF, Harris-Kawano A, Perez D, Saeed Z, Hoag B, Jain R, Evans-Molina C, DiMeglio LA, Ismail HM, Dabelea D, Johnson RK, Urazbayeva M, Wentworth JM, Griffin KJ, Sims EK; ADA/EASD PMDI. Felton JL, et al. Commun Med (Lond). 2024 Apr 6;4(1):66. doi: 10.1038/s43856-024-00478-y. Commun Med (Lond). 2024. PMID: 38582818 Free PMC article.
References
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. - PubMed
- Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001 Diabetes. 2004;53:250–264. - PubMed
- Diabetes Control and Complications Trial Research Group: Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial: a randomized, controlled trial. Ann Intern Med. 1998;128:517–523. - PubMed
- Steffes MW, Sibley S, Jackson M, Thomas W, Steffes MW, Sibley S, et al. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. - PubMed
- Feutren G, Assan R, Karsenty G, Du Rostu H, Sirmai J, Papoz L, et al. Cyclosporin increases the rate and length of remission in insulin-dependent diabetes of recent onset: results of a multicentre double-blind trial. Lancet. 1986;328:119–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR000400/RR/NCRR NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- UL1 RR031986/RR/NCRR NIH HHS/United States
- U01 DK085463/DK/NIDDK NIH HHS/United States
- U01 DK061055/DK/NIDDK NIH HHS/United States
- U01 DK061036/DK/NIDDK NIH HHS/United States
- UL1 TR001085/TR/NCATS NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK061041/DK/NIDDK NIH HHS/United States
- UC4 DK117009/DK/NIDDK NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- U01 DK084565/DK/NIDDK NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 DK061040/DK/NIDDK NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- UL1 RR029890/RR/NCRR NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- U01 DK085505/DK/NIDDK NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- M01 RR00400/RR/NCRR NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- U01 DK061016/DK/NIDDK NIH HHS/United States
- UL1 RR025744/RR/NCRR NIH HHS/United States
- HHSN267200800019C/DK/NIDDK NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous