Adiponectin receptor signalling in the brain - PubMed (original) (raw)
Review
Adiponectin receptor signalling in the brain
John Thundyil et al. Br J Pharmacol. 2012 Jan.
Abstract
Adiponectin is an important adipocyte-derived hormone that regulates metabolism of lipids and glucose, and its receptors (AdipoR1, AdipoR2, T-cadherin) appear to exert actions in peripheral tissues by activating the AMP-activated protein kinase, p38-MAPK, PPARα and NF-kappa B. Adiponectin has been shown to exert a wide range of biological functions that could elicit different effects, depending on the target organ and the biological milieu. There is substantial evidence to suggest that adiponectin receptors are expressed widely in the brain. Their expression has been detected in regions of the mouse hypothalamus, brainstem, cortical neurons and endothelial cells, as well as in whole brain and pituitary extracts. While there is now considerable evidence for the presence of adiponectin and its receptors in the brain, their precise roles in brain diseases still remain unclear. Only a few research studies have looked at this facet of adiponectins in brain disorders. This brief review will describe the evidence for important functions by adiponectin, its structure and known actions, evidence for expression of AdipoRs in the brain, their involvement in brain disorders and the therapeutic potential of agents that could modify AdipoR signalling.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
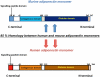
Figure 1
Schematic representation of adiponectin isoforms. The human and murine isoforms of adiponectin have 85% structural homology. Each of the adiponectin isoforms is composed of an N-terminal collagenous domain and a C-terminal globular region.
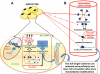
Figure 2
Regulation of synthesis, secretion and circulation of adiponectin. (A) Adipocytes synthesize adiponectin mRNA in its monomeric form within the nucleus. This transcription is regulated and promoted by SIRT1/FOXO-1 and PPARα. Once transcribed, the adiponectin protein monomer is released into the ER, where it undergoes various post-translational modifications, regulated by ER chaperones like ERp44 and ErO1- Lα to form trimers, hexamers and HMW (full-length adiponectin) isoforms. (B) Following their packaging in the golgi, the adiponectin isomers are released into the peripheral circulation. The HMW isomer is the most abundant and biologically active form of adioponectin. Another form of circulating adiponectin is the gAD leukocyte elastase-mediated cleavage of the globular domain of the trimeric adiponectin.
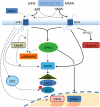
Figure 3
Representation of signalling transduction via adiponectin receptor activation. Adiponectin exists in full-length, globular, high molecular and low molecular weight forms. The binding of the different forms of adiponectin to the two known adiponectin receptors, AdipoR1 and AdipoR2, can lead to stimulation of AMPK, p38-MAPK, JNK and PPARα. Interacting directly with the N-terminal of adiponectin receptors, APPL1 elicits signalling through PPARα and along with AMPK modulates the PI3K pathway and eNOS levels. ACC phosphorylation occurs from CK2 adaptor protein at the AdipoR1 N-terminus whilst interaction of the scaffold protein RACK-I at AdipoR1 can mediate glucose metabolism. The ER chaperone, ERp46 helps mediate AdipoR1 signalling to p38 and AMPK. Signalling to NF-κB is solely through AdipoR1, but evidence exists for both its activation and inhibition upon gAD binding. Activation of both receptors results in activation of the caspase cascade and alterations in cell survival.
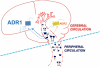
Figure 4
Differences between the peripheral and cerebral circulations regarding adiponectin signalling. The tight regulated membrane permeability of the cerebral blood vessels, as compared with their peripheral counterparts, permits the selective passage of only the trimers, hexamers and possibly globular forms of adiponectin into the CNS. Both AdipoR1and AdipoR2 are present in the brain, with AdipoR1 being more pronounced. Different adiponectin isomers bind to the AdipoRs with different binding affinities, as demonstrated by the thickness of the arrows.
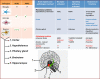
Figure 5
The CNS distribution of adiponectin receptors and their roles in adiponectin-mediated signalling in normal and disease states. Table A indicates the different cells of the CNS and their AdipoR receptor expression patterns. Expression of AdipoRs in isolated microglia is yet to be ascertained. The neuronal expression of both AdipoR1 and AdipoR2 has been demonstrated in different brain regions as indicated in the figure. Table B indicates the roles of adiponectin-mediated signalling.
Similar articles
- Adiponectin Is Involved in Connective Tissue Growth Factor-Induced Proliferation, Migration and Overproduction of the Extracellular Matrix in Keloid Fibroblasts.
Luo L, Li J, Liu H, Jian X, Zou Q, Zhao Q, Le Q, Chen H, Gao X, He C. Luo L, et al. Int J Mol Sci. 2017 May 12;18(5):1044. doi: 10.3390/ijms18051044. Int J Mol Sci. 2017. PMID: 28498357 Free PMC article. - Role of Adiponectin in Central Nervous System Disorders.
Bloemer J, Pinky PD, Govindarajulu M, Hong H, Judd R, Amin RH, Moore T, Dhanasekaran M, Reed MN, Suppiramaniam V. Bloemer J, et al. Neural Plast. 2018 Jul 29;2018:4593530. doi: 10.1155/2018/4593530. eCollection 2018. Neural Plast. 2018. PMID: 30150999 Free PMC article. Review. - A Saccharomyces cerevisiae assay system to investigate ligand/AdipoR1 interactions that lead to cellular signaling.
Aouida M, Kim K, Shaikh AR, Pardo JM, Eppinger J, Yun DJ, Bressan RA, Narasimhan ML. Aouida M, et al. PLoS One. 2013 Jun 7;8(6):e65454. doi: 10.1371/journal.pone.0065454. Print 2013. PLoS One. 2013. PMID: 23762377 Free PMC article. - Tissue-specific downregulation of the adiponectin "system": possible implications for fat accumulation tendency in the pig.
De Rosa A, Monaco ML, Nigro E, Scudiero O, D'Andrea M, Pilla F, Oriani G, Daniele A. De Rosa A, et al. Domest Anim Endocrinol. 2013 Apr;44(3):131-8. doi: 10.1016/j.domaniend.2012.11.001. Epub 2012 Dec 3. Domest Anim Endocrinol. 2013. PMID: 23291014 - Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors.
Heiker JT, Kosel D, Beck-Sickinger AG. Heiker JT, et al. Biol Chem. 2010 Sep;391(9):1005-18. doi: 10.1515/BC.2010.104. Biol Chem. 2010. PMID: 20536390 Review.
Cited by
- Adiponectin and Inflammatory Marker Levels in the Elderly Patients with Diabetes, Mild Cognitive Impairment and Depressive Symptoms.
Gorska-Ciebiada M, Ciebiada M. Gorska-Ciebiada M, et al. Int J Mol Sci. 2024 Oct 8;25(19):10804. doi: 10.3390/ijms251910804. Int J Mol Sci. 2024. PMID: 39409133 Free PMC article. - The Role of Adipose Tissue and Nutrition in the Regulation of Adiponectin.
Baldelli S, Aiello G, Mansilla Di Martino E, Campaci D, Muthanna FMS, Lombardo M. Baldelli S, et al. Nutrients. 2024 Jul 26;16(15):2436. doi: 10.3390/nu16152436. Nutrients. 2024. PMID: 39125318 Free PMC article. Review. - Fucoidan Improves D-Galactose-Induced Cognitive Dysfunction by Promoting Mitochondrial Biogenesis and Maintaining Gut Microbiome Homeostasis.
Xu Y, Xue M, Li J, Ma Y, Wang Y, Zhang H, Liang H. Xu Y, et al. Nutrients. 2024 May 17;16(10):1512. doi: 10.3390/nu16101512. Nutrients. 2024. PMID: 38794753 Free PMC article. - Running exercise alleviates hippocampal neuroinflammation and shifts the balance of microglial M1/M2 polarization through adiponectin/AdipoR1 pathway activation in mice exposed to chronic unpredictable stress.
Liu L, Tang J, Liang X, Li Y, Zhu P, Zhou M, Qin L, Deng Y, Li J, Wang Y, Jiang L, Huang D, Zhou Y, Wang S, Xiao Q, Luo Y, Tang Y. Liu L, et al. Mol Psychiatry. 2024 Jul;29(7):2031-2042. doi: 10.1038/s41380-024-02464-1. Epub 2024 Feb 15. Mol Psychiatry. 2024. PMID: 38361125
References
- Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55(Suppl. 2):S145–S154. - PubMed
- Akifusa S, Kamio N, Shimazaki Y, Yamaguchi N, Yamashita Y. Regulation of globular adiponectin-induced apoptosis by reactive oxygen/nitrogen species in RAW264 macrophages. Free Radic Biol Med. 2008;45:1326–1339. - PubMed
- Alberti L, Gilardini L, Girola A, Moro M, Cavagnini F, Invitti C. Adiponectin receptors gene expression in lymphocytes of obese and anorexic patients. Diabetes Obes Metab. 2007;9:344–349. - PubMed
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases