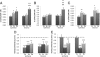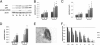The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice - PubMed (original) (raw)
doi: 10.1016/j.ajpath.2011.04.020. Epub 2011 Jun 12.
Carole Nicco, Charlotte Ngô, Christiane Chéreau, Sandrine Chouzenoux, Wioleta Marut, Jean Guibourdenche, Sylviane Arkwright, Bernard Weill, Charles Chapron, Bertrand Dousset, Frédéric Batteux
Affiliations
- PMID: 21718677
- PMCID: PMC3157265
- DOI: 10.1016/j.ajpath.2011.04.020
The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice
Mahaut Leconte et al. Am J Pathol. 2011 Aug.
Abstract
Deep infiltrating endometriosis (DIE) is a particular clinical and histological entity of endometriosis responsible for chronic pelvic pain and infertility. Here we characterize the proliferative phenotype of DIE cells, to explore the cellular and molecular mechanisms that could explain their aggressive potential. In addition, the inhibition of mTOR/AKT pathway was tested, as a potential treatment of DIE. Included were 22 patients with DIE and 12 control patients without endometriosis. Epithelial and stromal cells were extracted from biopsies of eutopic endometrium and deep infiltrating endometriotic nodules from patients with DIE. Cell proliferation was determined by thymidine incorporation. Oxidative stress was assayed by spectrofluorometry. The ERK and mTOR/AKT pathways were analyzed in vitro by Western blot and for AKT in vivo in a mouse model of DIE. The proliferation rate of eutopic endometrial cells and of deep infiltrating endometriotic cells from DIE patients was higher than that of endometrial cells from controls. The hyperproliferative phenotype of endometriotic cells was associated with an increase in endogenous oxidative stress, and with activation of the ERK and mTOR/AKT pathways. mTOR/AKT inhibition by temsirolimus decreased endometriotic cell proliferation both in vitro and in vivo in a mouse model of DIE. Blocking the mTOR/AKT pathway offers new prospects for the treatment of DIE.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
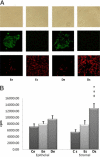
Purification and proliferative rates of endometriotic cells. A: Eutopic endometrial epithelial cells (Ee), eutopic endometrial stromal cells (Es), deep infiltrating endometriotic epithelial cells (De), and deep infiltrating endometriotic stromal cells (Ds) examined by contrast phase microscopy (top row), immunofluorescence with fluorescein isothiocyanate anti-cytokeratin antibodies (middle row), or Cy3 anti-vimentin antibodies (bottom row). Original magnification, ×100. B: Basal proliferative rate was assessed in the various cell lines: control endometrial epithelial cell lines (Ce), n = 11; Ee cell lines, n = 18; De cell lines, n = 17; control endometrial stromal cell lines (Cs), n = 10; Es cell lines, n = 18; and Ds cell lines, n = 18. Cell proliferation was determined by thymidine incorporation. Results are expressed as counts per minute (cpm). *P < 0.001, Es versus Ce cells; †P < 0.01, De versus Ee cells; ‡P < 0.05, stromal versus epithelial cells within the same endometrial eutopic or deep infiltrating lesion.
Figure 2
A–C: Cellular production of ROS and detoxification of H2O2 by N_-acetyl-L-cysteine (NAC). Basal intracellular levels of O2•− (A), H2O2 (B), and NO (C) were assessed by spectrofluorometry in the various cell lines (11 Ce, 18 Ee, 17 De, 10 Cs, 18 Es, and 18 Ds). U.A., arbitrary fluorescence intensity units. *P < 0.05, **P < 0.01, and ***P < 0.001, Es versus Ce cells. ††_P < 0.01, †††P < 0.001, De versus Ee cells. ‡‡P < 0.01, stromal versus epithelial cells within the same endometrial eutopic or deep infiltrating lesion. D and E: Intracellular levels of H2O2 (D) and proliferative rate (E) were assessed in cell lines treated with 6.4 mmol/L NAC versus untreated cells. The level in untreated cells is indicated by a horizontal dashed line. **P < 0.01, ***P < 0.001.
Figure 3
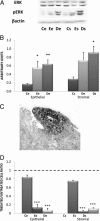
Exploration of the ERK pathway. A: Determination of ERK and pERK by Western blot in cell lysates. Western blotting of ERK and pERK were performed on lysates of the various cell lines (11 Ce, 18 Ee, 17 De, 10 Cs, 18 Es, and 18 Ds) using specific anti-ERK and anti-pERK antibodies. A representative Western blot is shown, obtained with all cell lines extracted from one patient and from one control. B: Quantitative analysis of ERK and pERK. Quantitative analysis of ERK and pERK was performed in the various cell lines (11 Ce, 18 Ee, 17 De, 10 Cs, 18 Es, and 18 Ds) by Western blot analysis. The mean optical density ratio for pERK/ERK was calculated in each endometriotic cell type and compared with control endometrial cells: *P < 0.05, **P < 0.01. C: IHC expression of pERK on deep infiltrating endometriotic tissue. A representative specimen is shown, from one of three patients. pERK-positive cells appear black. Original magnification, ×400. D: Effect of A77-1726 on endometriotic cell proliferation. Proliferative rates were determined by thymidine incorporation in the various cell lines (11 Ce, 18 Ee, 17 De, 10 Cs, 18 Es, and 18 Ds) treated with 200 μmol/L A77-1726 and were compared with untreated cells. The level in untreated cells is indicated by a horizontal dashed line. ***P < 0.001.
Figure 4
Exploration of the mTOR/AKT pathway. A: Determination of AKT, pAKT, and phospo-p70S6K (serine 371/threonine 389) by Western blot in cell lysates. Western blotting of AKT, pAKT, phospo-p70S6K, and β-actin was performed on lysates of the various cell lines (11 Ce, 18 Ee, 17 De, 10 Cs, 18 Es, and 18 Ds) using specific anti-AKT, anti-pAKT, anti-phospo-p70S6K, and anti-β-actin antibodies. The figure represents one representative Western blot obtained with all cell lines extracted from one patient and from one control. B and C: Quantitative analysis of AKT (B) and pAKT (C) was performed in the various cell lines (11 Ce, 18 Ee, 17 De, 10 Cs, 18 Es, and 18 Ds) by Western blot analysis. The mean optical density ratios AKT/β-actin and pAKT/β-actin were calculated in the various endometriotic cell lines. *P < 0.05 and **P < 0.001, endometriotic versus control cells; †P < 0.05, stromal versus epithelial cells within the same endometrial eutopic or deep infiltrating lesion. D: Quantitative analysis of phospo-p70S6K was performed in the various cell lines (11 Ce, 18 Ee, 17 De, 10 Cs, 18 Es, and 18 Ds) by Western blot analysis. The mean optical density ratio phospo-p70S6K/β-actin was calculated in the various endometriotic cell lines. *P < 0.05, endometriotic versus control cells. Stromal versus epithelial cells within the same endometrial eutopic or deep infiltrating lesion: †P < 0.05. E: IHC expression of pAKT on deep infiltrating endometriotic tissue. A representative specimen is shown, from one of three patients. pAKT-positive cells appear black. Original magnification, ×400. F: Effect of temsirolimus on proliferation. Proliferative rate was determined by thymidine incorporation for the various stromal cell lines (10 Cs, 18 Es, and 18 Ds) treated in vitro with increasing concentrations (0.3 to 24 μmol/L) of temsirolimus. The basal level of proliferation of untreated endometriotic cells is indicated by a horizontal dashed line. *P < 0.05, treated versus untreated cells.
Figure 5
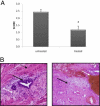
Effect of temsirolimus on deep infiltrating endometriotic tissue in a mouse model. A: Pathology scores were calculated on day 21 on untreated and treated mice: *P < 0.01. B: Histological views of deep infiltrating implant on day 21 in untreated mice (left) and in treated mice (right). In untreated mice, the appearance of the implanted deep infiltrating endometriotic tissue was typical, with endometriotic glands (black arrow) and with stroma (white arrow) surrounded by fibrosis and hyperplastic smooth muscle fibers (dotted black arrow). By contrast, treated mice (right) displayed fibrotic and avascular lesions (black arrow) with hemosiderin (white arrow).
Similar articles
- Co-operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro.
Matsuzaki S, Darcha C. Matsuzaki S, et al. Hum Reprod. 2015 Jul;30(7):1606-16. doi: 10.1093/humrep/dev108. Epub 2015 May 14. Hum Reprod. 2015. PMID: 25976656 - Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis.
Leconte M, Nicco C, Ngô C, Arkwright S, Chéreau C, Guibourdenche J, Weill B, Chapron C, Dousset B, Batteux F. Leconte M, et al. Am J Pathol. 2010 Dec;177(6):2963-70. doi: 10.2353/ajpath.2010.100375. Epub 2010 Nov 5. Am J Pathol. 2010. PMID: 21057002 Free PMC article. - Protein kinase inhibitors can control the progression of endometriosis in vitro and in vivo.
Ngô C, Nicco C, Leconte M, Chéreau C, Arkwright S, Vacher-Lavenu MC, Weill B, Chapron C, Batteux F. Ngô C, et al. J Pathol. 2010 Oct;222(2):148-57. doi: 10.1002/path.2756. J Pathol. 2010. PMID: 20821752 - Pathogenetic Mechanisms of Deep Infiltrating Endometriosis.
Tosti C, Pinzauti S, Santulli P, Chapron C, Petraglia F. Tosti C, et al. Reprod Sci. 2015 Sep;22(9):1053-9. doi: 10.1177/1933719115592713. Epub 2015 Jul 12. Reprod Sci. 2015. PMID: 26169038 Review. - Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics.
McKinnon BD, Kocbek V, Nirgianakis K, Bersinger NA, Mueller MD. McKinnon BD, et al. Hum Reprod Update. 2016 Apr;22(3):382-403. doi: 10.1093/humupd/dmv060. Epub 2016 Jan 5. Hum Reprod Update. 2016. PMID: 26740585 Review.
Cited by
- Everolimus as an mTOR Inhibitor Suppresses Endometriotic Implants: an Experimental Rat Study.
Kacan T, Yildiz C, Baloglu Kacan S, Seker M, Ozer H, Cetin A. Kacan T, et al. Geburtshilfe Frauenheilkd. 2017 Jan;77(1):66-72. doi: 10.1055/s-0042-115566. Geburtshilfe Frauenheilkd. 2017. PMID: 28190891 Free PMC article. - The cannabinoid receptor CB1 affects the proliferation and apoptosis of adenomyotic human uterine smooth muscle cells of the junctional zone: a mechanism study.
Wang S, Li B, Shen X, Duan H, Guo Z, Li X, Sun F. Wang S, et al. Reprod Biol Endocrinol. 2021 Feb 2;19(1):16. doi: 10.1186/s12958-020-00690-0. Reprod Biol Endocrinol. 2021. PMID: 33531043 Free PMC article. - Chronic pelvic pain in endometriosis: an overview.
Triolo O, Laganà AS, Sturlese E. Triolo O, et al. J Clin Med Res. 2013 Jun;5(3):153-63. doi: 10.4021/jocmr1288w. Epub 2013 Apr 23. J Clin Med Res. 2013. PMID: 23671540 Free PMC article. - Role of mTOR Signaling in Female Reproduction.
Guo Z, Yu Q. Guo Z, et al. Front Endocrinol (Lausanne). 2019 Oct 9;10:692. doi: 10.3389/fendo.2019.00692. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31649622 Free PMC article. Review. - Inhibitory Effects of 6,8-Diprenylorobol on Endometriosis Progression in Humans by Disrupting Calcium Homeostasis and Mitochondrial Function.
Song J, Song G, Park S, Lim W. Song J, et al. Antioxidants (Basel). 2022 Jan 17;11(1):171. doi: 10.3390/antiox11010171. Antioxidants (Basel). 2022. PMID: 35052675 Free PMC article.
References
- Giudice L.C., Kao L.C. Endometriosis. Lancet. 2004;364:1789–1799. - PubMed
- Chapron C., Fauconnier A., Dubuisson J.B., Barakat H., Vieira M., Bréart G. Deep infiltrating endometriosis: relation between severity of dysmenorrhoea and extent of disease. Hum Reprod. 2003;18:760–766. - PubMed
- Laursen B.S., Bajaj P., Olesen A.S., Delmar C., Arendt-Nielsen L. Health related quality of life and quantitative pain measurement in females with chronic non-malignant pain. Eur J Pain. 2005;9:267–275. - PubMed
- de Ziegler D., Borghese B., Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–738. - PubMed
- Chopin N., Vieira M., Borghese B., Foulot H., Dousset B., Coste J., Mignon A., Fauconnier A., Chapron C. Operative management of deeply infiltrating endometriosis: results on pelvic pain symptoms according to a surgical classification. J Minim Invasive Gynecol. 2005;12:106–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous