The poly(ADP-Ribose) polymerase inhibitor ABT-888 reduces radiation-induced nuclear EGFR and augments head and neck tumor response to radiotherapy - PubMed (original) (raw)
The poly(ADP-Ribose) polymerase inhibitor ABT-888 reduces radiation-induced nuclear EGFR and augments head and neck tumor response to radiotherapy
Somaira Nowsheen et al. Radiother Oncol. 2011 Jun.
Abstract
Background and purpose: Current therapies for head and neck cancer frequently are not curative, necessitating novel therapeutic strategies. Thus, we studied whether inhibition of poly(ADP-Ribose) polymerase (PARP), a key DNA repair enzyme, could improve efficacy of radiotherapy in human head and neck cancer.
Materials and methods: UM-SCC1, UM-SCC5, UM-SCC6, and FaDu human head and neck cancer cellular susceptibility to the PARP inhibitor (PARPi) ABT-888 and/or radiation (IR) was assessed using colony formation assays. DNA damage was evaluated using the alkaline comet assay and immunostaining for γ-H2AX foci. Non-homologous end-joining (NHEJ) mediated repair was measured using phospho-DNA-Pk foci. Epidermal growth factor receptor (EGFR) location was assessed by immunostaining. Poly ADP-Ribose polymerization (PAR) levels were assessed using immunoblotting.
Results: Human head and neck cancer cells exhibited enhanced cytotoxicity with IR and ABT-888 compared to either agent alone. This increased susceptibility correlated with reduced nuclear EGFR, attenuation of NHEJ, and persistence of DNA damage following IR. Interestingly, a subset of head and neck cancer cells which had elevated basal PAR levels was susceptible to PARPi alone.
Conclusions: Combining radiotherapy and PARP inhibition may improve outcomes and quality of life for head and neck cancer patients treated with radiotherapy. Furthermore, this novel strategy may also be feasible in other tumor types. Moreover, PAR levels should be investigated as a potential biomarker for tumor susceptibility to PARP inhibition.
Published by Elsevier Ireland Ltd.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflict of interest.
Figures
FIGURE 1. IR augments head and neck tumor susceptibility to the PARP inhibitor ABT-888
Combination of IR and ABT-888 reduces the viability of (A) UM-SCC1, (B) UM-SCC5, (C) UM-SCC6, and (D) FaDu head and neck cancer cells. Shown is the representative data of at least 3 independent experiments of the cell viability following various treatments as measured by colony formation assay, corrected for the survival fraction following RT (mean +/− SEM, *p<0.01, **p<0.001). Interestingly, UM-SCC1 and UM-SCC5 demonstrate susceptibility to ABT-888 alone.
FIGURE 2. Persistent DNA damage is observed in head and neck cancer cells treated with ABT-888 and radiation
The inset in (A) is a representative image of UM-SCC1 cells exhibiting comet tail following IR. IR enhances the mean tail moment, indicative of total DNA damage (SSB and DSB) in ABT-888 treated (B) UM-SCC1 and (C) UM-SCC6 cell lines, as measured by the alkaline comet assay. Addition of ABT-888 inhibits resolution of IR-induced DNA damage. The inset in (D) is a representative image of UM-SCC1 cells exhibiting γ-H2AX foci, a commonly used marker for DSBs, following IR. The comet data correlates with the number of cells with DSBs in (E) UM-SCC1 and (F) UM-SCC6 cells as evidenced by persistent γ-H2AX foci. Shown is the representative data of 3 independent experiments the % of cells (mean +/− SEM) with >10 foci (*p<0.05, **p<0.01).
FIGURE 3. ABT-888 attenuates IR-induced non-homologous end joining (NHEJ) in head and neck cancer cells
The inset in (A) is a representative image of UM-SCC1 cells exhibiting DNA Pk T2609 foci, well characterized markers of NHEJ-mediated DNA DSB repair, following IR. ABT-888 attenuates IR-induced phosphorylated DNA Pk foci in (B) UM-SCC1, (C) UM-SCC5, (D) UM-SCC6, and (E) FaDu cells. Shown is the representative data of 3 independent experiments the % of cells (mean +/− SEM) with >10 foci (*p<0.05, **p<0.01).
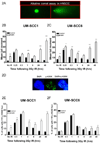
FIGURE 4. ABT-888 reduces nuclear epidermal growth factor receptor (EGFR) following radiation
The inset in (A) is a representative image of UM-SCC6 cells exhibiting nuclear (N), both nuclear and cytosolic (NC), and cytosolic (C) EGFR. EGFR location was analyzed following IR and cells were assessed as having predominantly nuclear staining, predominantly cytoplasmic staining, or mixed nuclear/cytoplasmic staining. ABT-888 blocks nuclear translocation of EGFR following IR in (B) UM-SCC1, (C) UM-SCC5, (D) UM-SCC6, and (E) FaDu cells. Shown is the representative data of three independent experiments the % of cells (mean ± SEM) with strictly nuclear (N), strictly cytosolic (C), or mixed nuclear/cytosolic (NC) EGFR staining (*p < 0.05, **p < 0.01).
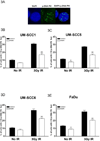
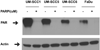
FIGURE 5. Basal PAR level correlates with susceptibility of head and neck cancer cells to ABT-888
Basal PAR levels in head and neck cells were analyzed following ABT-888 treatment and correlated with susceptibility to ABT-888. Shown is a representative western blot of at least 3 independent experiments.
Similar articles
- Cetuximab augments cytotoxicity with poly (adp-ribose) polymerase inhibition in head and neck cancer.
Nowsheen S, Bonner JA, Lobuglio AF, Trummell H, Whitley AC, Dobelbower MC, Yang ES. Nowsheen S, et al. PLoS One. 2011;6(8):e24148. doi: 10.1371/journal.pone.0024148. Epub 2011 Aug 30. PLoS One. 2011. PMID: 21912620 Free PMC article. - Linifanib (ABT-869), enhances cytotoxicity with poly (ADP-ribose) polymerase inhibitor, veliparib (ABT-888), in head and neck carcinoma cells.
Hsu HW, de Necochea-Campion R, Williams V, Duerksen-Hughes PJ, Simental AA Jr, Ferris RL, Chen CS, Mirshahidi S. Hsu HW, et al. Oral Oncol. 2014 Jul;50(7):662-9. doi: 10.1016/j.oraloncology.2014.03.006. Epub 2014 Apr 13. Oral Oncol. 2014. PMID: 24735547 - Combined EGFR1 and PARP1 Inhibition Enhances the Effect of Radiation in Head and Neck Squamous Cell Carcinoma Models.
Frederick BA, Gupta R, Atilano-Roque A, Su TT, Raben D. Frederick BA, et al. Radiat Res. 2020 Nov 10;194(5):519-531. doi: 10.1667/RR15480.1. Radiat Res. 2020. PMID: 32936912 Free PMC article. - Targeting poly (ADP) ribose polymerase I (PARP-1) and PARP-1 interacting proteins for cancer treatment.
Sakamoto-Hojo ET, Balajee AS. Sakamoto-Hojo ET, et al. Anticancer Agents Med Chem. 2008 May;8(4):402-16. doi: 10.2174/187152008784220302. Anticancer Agents Med Chem. 2008. PMID: 18473725 Review. - Epidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancers.
Herbst RS, Langer CJ. Herbst RS, et al. Semin Oncol. 2002 Feb;29(1 Suppl 4):27-36. doi: 10.1053/sonc.2002.31525. Semin Oncol. 2002. PMID: 11894011 Review.
Cited by
- PARylation of GCN5 by PARP1 mediates its recruitment to DSBs and facilitates both HR and NHEJ Repair.
Sarkar D, Chakraborty A, Mandi S, Dutt S. Sarkar D, et al. Cell Mol Life Sci. 2024 Nov 7;81(1):446. doi: 10.1007/s00018-024-05469-9. Cell Mol Life Sci. 2024. PMID: 39508866 - PARP inhibitors combined with radiotherapy: are we ready?
Sun C, Chu A, Song R, Liu S, Chai T, Wang X, Liu Z. Sun C, et al. Front Pharmacol. 2023 Oct 26;14:1234973. doi: 10.3389/fphar.2023.1234973. eCollection 2023. Front Pharmacol. 2023. PMID: 37954854 Free PMC article. Review. - Recent findings on the impact of ErbB receptors status on prognosis and therapy of head and neck squamous cell carcinoma.
Palumbo C, Benvenuto M, Focaccetti C, Albonici L, Cifaldi L, Rufini A, Nardozi D, Angiolini V, Bei A, Masuelli L, Bei R. Palumbo C, et al. Front Med (Lausanne). 2023 Feb 2;10:1066021. doi: 10.3389/fmed.2023.1066021. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36817764 Free PMC article. Review. - Radiotherapy and the cellular DNA damage response: current and future perspectives on head and neck cancer treatment.
Fabbrizi MR, Parsons JL. Fabbrizi MR, et al. Cancer Drug Resist. 2020 Sep 17;3(4):775-790. doi: 10.20517/cdr.2020.49. eCollection 2020. Cancer Drug Resist. 2020. PMID: 35582232 Free PMC article. Review. - An open-label, pilot study of veliparib and lapatinib in patients with metastatic, triple-negative breast cancer.
Stringer-Reasor EM, May JE, Olariu E, Caterinicchia V, Li Y, Chen D, Della Manna DL, Rocque GB, Vaklavas C, Falkson CI, Nabell LM, Acosta EP, Forero-Torres A, Yang ES. Stringer-Reasor EM, et al. Breast Cancer Res. 2021 Mar 4;23(1):30. doi: 10.1186/s13058-021-01408-9. Breast Cancer Res. 2021. PMID: 33663560 Free PMC article. Clinical Trial.
References
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. - PubMed
- Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. - PubMed
- Donawho CK, Luo Y, Luo Y, et al. ABT-888, an Orally Active Poly(ADP-Ribose) Polymerase Inhibitor that Potentiates DNA-Damaging Agents in Preclinical Tumor Models. Clinical Cancer Research. 2007;13:2728–2737. - PubMed
- Peter RU, Beetz A, Ried C, Michel G, van Beuningen D, Ruzicka T. Increased expression of the epidermal growth factor receptor in human epidermal keratinocytes after exposure to ionizing radiation. Radiat Res. 1993;136:65–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous