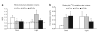Impact of high-fat diet and obesity on energy balance and fuel utilization during the metabolic challenge of lactation - PubMed (original) (raw)
Impact of high-fat diet and obesity on energy balance and fuel utilization during the metabolic challenge of lactation
Jessica L Wahlig et al. Obesity (Silver Spring). 2012 Jan.
Abstract
The effects of obesity and a high-fat (HF) diet on whole body and tissue-specific metabolism of lactating dams and their offspring were examined in C57/B6 mice. Female mice were fed low-fat (LF) or HF diets before and throughout pregnancy and lactation. HF-fed mice were segregated into lean (HF-Ln) and obese (HF-Ob) groups before pregnancy by their weight gain response. Compared to LF-Ln dams, HF-Ln, and HF-Ob dams exhibited a greater positive energy balance (EB) and increased dietary fat retention in peripheral tissues (P < 0.05). HF-Ob dams had greater dietary fat retention in liver and adipose compared to HF-Ln dams (P < 0.05). De novo synthesized fat was decreased in tissues and milk from HF-fed dams compared to LF-Ln dams (P < 0.05). However, less dietary and de novo synthesized fat was found in the HF-Ob mammary glands compared to HF-Ln (P < 0.05). Obesity was associated with reduced milk triglycerides relative to lean controls (P < 0.05). Compared to HF diet alone obesity has additional adverse affects, impairing both lipid metabolism as well as milk fat production. Growth rates of LF-Ln litters were lower than HF-Ln and HF-Ob litters (P < 0.05). Total energy expenditure (TEE) of HF-Ob litters was reduced relative to HF-Ln litters, whereas their respiratory exchange ratios (RERs) were increased (P < 0.05). Collectively these data show that consumption of a HF diet significantly affects maternal and neonatal metabolism and that maternal obesity can independently alter these responses.
Conflict of interest statement
Disclosure: The authors declared no conflict of interest.
Figures
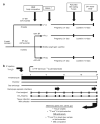
Figure 1
Experimental design for feeding paradigm and dual-tracer study. The overall study design and feeding paradigm are shown in a. In addition, a timeline for mating, pregnancy, and lactation are included. The dual-tracer study is depicted in b where a metabolic monitoring chamber was used to assess the fuel utilization and lipid trafficking of exogenous fat in lactating mice.
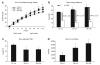
Figure 2
Maternal energetics and activity. Cumulative energy intake (EI), energy balance, and activity were measured over the 24-h study and expressed as means ± s.e. Bars with a single asterisk are significantly different from low-fat lean dam (LF-Ln) controls and bars with a double asterisk are significantly different from high-fat lean dam (HF-Ln) controls (P < 0.05). (a) Cumulative energy intake was measured every 3 h over a 24-h period and expressed in kcal. (b) Energy balance showed as the difference between total energy expenditure (TEE) and total energy intake (EI) over 24-h and expressed as kcal/day (†LF-Ln vs. HF-Ln or HF-Ob, P < 0.05). (c) Adjusted total energy expenditure was examined by analysis of covariance, using lean mass as the covariate and expressed as kcal/24 h. (d) 24-h activity was measured using infrared laser beams. Each beam break was considered one activity count.
Figure 3
Maternal whole body fuel utilization and dietary fat oxidation. The oxidation of dietary fat and whole body fuel utilization measured during dark and light cycles are expressed as means ± s.e. Bars with a single asterisk are significantly different from low-fat lean dam (LF-Ln) controls and bars with a double asterisk are significantly different from high-fat lean dam (HF-Ln) controls (P < 0.05). (a) Respiratory exchange ratio (RER; CO2/O2) derived from indirect calorimetry measurements. (b) Oxidation of dietary fat was assessed by measuring 14C-CO2 in expired air over 4.5 min at each time point.
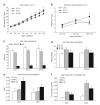
Figure 4
Analysis of litter energy expenditure and fuel utilization. All values are expressed as a mean ± s.e. Bars with a single asterisk are significantly different from low-fat lean dam (LF-Ln) controls and bars with a double asterisk are significantly different from high-fat lean dam (HF-Ln) controls (P < 0.05). (a) Growth curve of litters normalized to five pups over the first 10 days of lactation. (b) Daily litter weight change of litters normalized to five pups between days L2 to L3, L5 to L6, and L9 to L10. (c) Litter body composition on the study day depicted as total body weight (BW), fat-free mass (FFM), and fat mass (FM). (d) Total energy expenditure (Raw) expressed over the 24 h as kcal/day; total energy expenditure (adjusted) was examined by analysis of covariance, using lean mass as the covariate. (e) Respiratory exchange ratio (RER; CO2/O2) derived from indirect calorimetry measurements is expressed during the dark and light cycle. (f) Oxidation of dietary fat was assessed by measuring 14C-CO2 in expired air over 4.5 min at each time point during the light and dark cycle.
Similar articles
- Maternal obesity reduces milk lipid production in lactating mice by inhibiting acetyl-CoA carboxylase and impairing fatty acid synthesis.
Saben JL, Bales ES, Jackman MR, Orlicky D, MacLean PS, McManaman JL. Saben JL, et al. PLoS One. 2014 May 21;9(5):e98066. doi: 10.1371/journal.pone.0098066. eCollection 2014. PLoS One. 2014. PMID: 24849657 Free PMC article. - Maternal obesity is necessary for programming effect of high-fat diet on offspring.
White CL, Purpera MN, Morrison CD. White CL, et al. Am J Physiol Regul Integr Comp Physiol. 2009 May;296(5):R1464-72. doi: 10.1152/ajpregu.91015.2008. Epub 2009 Feb 25. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19244583 Free PMC article. - Leucine supplementation in maternal high-fat diet alleviated adiposity and glucose intolerance of adult mice offspring fed a postweaning high-fat diet.
Kim J, Kim J, Kwon YH. Kim J, et al. Lipids Health Dis. 2023 Apr 15;22(1):50. doi: 10.1186/s12944-023-01812-4. Lipids Health Dis. 2023. PMID: 37061742 Free PMC article. - Limits to sustained energy intake. XXVIII. Beneficial effects of high dietary fat on lactation performance in mice.
Kagya-Agyemang JK, Vaanholt LM, Hambly C, Król E, Mitchell SE, Speakman JR. Kagya-Agyemang JK, et al. J Exp Biol. 2018 Aug 23;221(Pt 16):jeb180828. doi: 10.1242/jeb.180828. J Exp Biol. 2018. PMID: 29941615 - Analysis of energy expenditure in diet-induced obese rats.
Assaad H, Yao K, Tekwe CD, Feng S, Bazer FW, Zhou L, Carroll RJ, Meininger CJ, Wu G. Assaad H, et al. Front Biosci (Landmark Ed). 2014 Jun 1;19(6):967-85. doi: 10.2741/4261. Front Biosci (Landmark Ed). 2014. PMID: 24896330 Free PMC article. Review.
Cited by
- Postnatal feeding with high-fat diet induces obesity and precocious puberty in C57BL/6J mouse pups: a novel model of obesity and puberty.
Ullah R, Su Y, Shen Y, Li C, Xu X, Zhang J, Huang K, Rauf N, He Y, Cheng J, Qin H, Zhou YD, Fu J. Ullah R, et al. Front Med. 2017 Jun;11(2):266-276. doi: 10.1007/s11684-017-0530-y. Epub 2017 May 13. Front Med. 2017. PMID: 28500430 - Serotonin Deficiency Rescues Lactation on Day 1 in Mice Fed a High Fat Diet.
Weaver SR, Bohrer JC, Prichard AS, Perez PK, Streckenbach LJ, Olson JM, Cook ME, Hernandez LL. Weaver SR, et al. PLoS One. 2016 Sep 7;11(9):e0162432. doi: 10.1371/journal.pone.0162432. eCollection 2016. PLoS One. 2016. PMID: 27603698 Free PMC article. - Maternal Supplementation with a Cocoa Extract during Lactation Deeply Modulates Dams' Metabolism, Increases Adiponectin Circulating Levels and Improves the Inflammatory Profile in Obese Rat Offspring.
Mariné-Casadó R, Domenech-Coca C, Crescenti A, Rodríguez Gómez MÁ, Del Bas JM, Arola L, Boqué N, Caimari A. Mariné-Casadó R, et al. Nutrients. 2022 Dec 3;14(23):5134. doi: 10.3390/nu14235134. Nutrients. 2022. PMID: 36501173 Free PMC article. - Reverting to a Healthy Diet during Lactation Normalizes Maternal Milk Lipid Content of Diet-Induced Obese Rats and Prevents Early Alterations in the Plasma Lipidome of the Offspring.
Castillo P, Kuda O, Kopecky J, Pomar CA, Palou A, Palou M, Picó C. Castillo P, et al. Mol Nutr Food Res. 2022 Sep;66(17):e2200204. doi: 10.1002/mnfr.202200204. Epub 2022 Jul 13. Mol Nutr Food Res. 2022. PMID: 35772018 Free PMC article. - Understanding the Link Between Maternal Overnutrition, Cardio-Metabolic Dysfunction and Cognitive Aging.
Peleg-Raibstein D. Peleg-Raibstein D. Front Neurosci. 2021 Feb 26;15:645569. doi: 10.3389/fnins.2021.645569. eCollection 2021. Front Neurosci. 2021. PMID: 33716660 Free PMC article. Review.
References
- Butte NF. Impact of infant feeding practices on childhood obesity. J Nutr. 2009;139:412S–416S. - PubMed
- Rasmussen KM. The influence of maternal nutrition on lactation. Annu Rev Nutr. 1992;12:103–117. - PubMed
- McNamara JP. Lipid metabolism in adipose tissue during lactation: a model of a metabolic control system. J Nutr. 1994;124:1383S–1391S. - PubMed
- Corpeleijn E, Saris WH, Blaak EE. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev. 2009;10:178–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21HD050863/HD/NICHD NIH HHS/United States
- TL1 RR025778/RR/NCRR NIH HHS/United States
- P30 DK048520/DK/NIDDK NIH HHS/United States
- R01 DK038088/DK/NIDDK NIH HHS/United States
- DK48520/DK/NIDDK NIH HHS/United States
- TL1 TR001081/TR/NCATS NIH HHS/United States
- P01 HD038129/HD/NICHD NIH HHS/United States
- L1RR025778/RR/NCRR NIH HHS/United States
- R01 HD045965/HD/NICHD NIH HHS/United States
- P0IHD38129/PHS HHS/United States
- R21 HD050863/HD/NICHD NIH HHS/United States
- DK038088/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous