Innervation patterns of autonomic axons in the human endocrine pancreas - PubMed (original) (raw)
Innervation patterns of autonomic axons in the human endocrine pancreas
Rayner Rodriguez-Diaz et al. Cell Metab. 2011.
Abstract
The autonomic nervous system regulates hormone secretion from the endocrine pancreas, the islets of Langerhans, thus impacting glucose metabolism. The parasympathetic and sympathetic nerves innervate the pancreatic islet, but the precise innervation patterns are unknown, particularly in human. Here we demonstrate that the innervation of human islets is different from that of mouse islets and does not conform to existing models of autonomic control of islet function. By visualizing axons in three dimensions and quantifying axonal densities and contacts within pancreatic islets, we found that, unlike mouse endocrine cells, human endocrine cells are sparsely contacted by autonomic axons. Few parasympathetic cholinergic axons penetrate the human islet, and the invading sympathetic fibers preferentially innervate smooth muscle cells of blood vessels located within the islet. Thus, rather than modulating endocrine cell function directly, sympathetic nerves may regulate hormone secretion in human islets by controlling local blood flow or by acting on islet regions located downstream.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
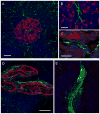
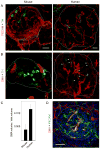
Figure 1. Human islets are sparsely innervated
(A and B) A mouse pancreatic section showing immunostaining for synapsin I/II (green) within an islet. Numerous axonal varicosities are present throughout the islet. Glucagon immunostaining (red) and nuclear counterstaining (DAPI, blue) are merged with synapsin labeling in (B) and (D). Unless otherwise stated, all figures in the article show maximal projections of z-stacks of 40 confocal images. Scale bar = 50 μm. (C and D) Synapsin immunostaining (green) in a human pancreatic section. Few immunostained axons can be seen in the islet and these are localized to discrete regions.
Figure 2. Axon immunostaining in the pancreas shows tissue specific staining patterns
(A) A human pancreatic section immunostained for synapsin (green) and glucagon (red in A-C) shows many synapsin-labeled axons in the exocrine tissue, but only few penetrating the islet. (B and C) Strong synapsin staining (green) can be seen inside the islet in large axons running along elongated cell nuclei. These axons do not enter the endocrine tissue (glucagon staining shown in red). (D and E) Strong synapsin staining (green) can be seen inside around large arteries (D, alpha smooth muscle actin in red) or in nerves traversing the exocrine pancreas (E). Scale bars = 50 μm (A, D, E; in D also applies to E), 20 μm (B), and 10 μm (C).
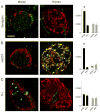
Figure 3. The innervation of the human islet is different from that of the mouse islet
(A) Synapsin I/II immunostaining (green) differs qualitatively and quantitatively between mouse and human islets. Maximal projections of confocal images show numerous axonal varicosities in mouse islets but few long axons in human islets. Quantification (right) shows that the density of immunostained axons is much higher in mouse endocrine (endo) regions than in exocrine regions (exo, black bars). There is no increased innervation density in the human endocrine pancreas (grey bars). Innervation density is expressed as the ratio of axonal labeling to total examined islet volume (see Experimental Procedures). One-way ANOVA followed by multiple comparisons (Student-Newman-Keuls). Data are presented as mean ± SEM. Glucagon staining (red) in all panels shows the extent of the islet. Scale bar = 50 μm (applies to all panels). (B) The patterns of vesicular acetylcholine transporter (vAChT) immunostaining (green) are completely different in mouse and human islets. Axonal varicosities fill the core of the mouse islet, whereas axons are absent in human islets. Notice that many cells are labeled in human islets. Quantification of axons (right) shows that the innervation density is highest in the mouse islet (endo). A quantification of vAChT-labeled axons in human islets was not possible due to strong cellular staining (ND = not determined). (C) Tyrosine hydroxylase (TH) immunostaining (green) is very different in mouse and human islets. TH-labeled fibers are localized to the mantle of the mouse islet. Large TH-labeled axons penetrate the human islet where they localize to discrete regions in the core of the islet. Notice that some mouse endocrine cells are also TH-immunostained. Quantification of the innervation densities (right) shows no differences between pancreatic regions and species.
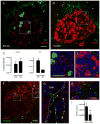
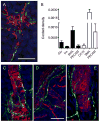
Figure 4. Cholinergic innervation of mouse and human islets
(A and B) Maximal projection of confocal images of a mouse (A) and a human pancreatic section (B) immunostained for the cholinergic marker vAChT (red) and the catecholaminergic marker TH (green). Notice the complementary staining patterns in the mouse islet. Arrows in B point at vAChT stained axons in the human exocrine tissue. Scale bar = 20 μm (applies to A and B). (C) Quantification of contacts between vAChT-labeled axons and endocrine cells in mouse islets. Cholinergic axons targeted glucagon-labeled alpha (Glu) and insulin-labeled beta cells (Ins) alike, whereas catecholaminergic axons preferentially contacted alpha cells. Contacts were automatically detected in three dimensions by Volocity software as “intersects” and were expressed as the ratio of the intersect volume to the volume of the respective endocrine cell immunostaining (contact density; see Experimental Procedures). The ratio is equivalent to the innervation density of each cell population. Data are presented as mean ± SEM. (D and E) Higher magnification images of islet regions delimited by the boxes shown in A and B. Whereas vAChT-labeled varicosities were dense in the mouse islet parenchyma (D), these could not be seen in regions devoid of cellular vAChT staining in the human islet (E). Scale bar = 20 μm (applies to D and E). (F–H) Double labeling of vAChT (red) with synapsin (green) revealed the presence of cholinergic axons in the human pancreas. Arrows point at vAChT stained axons in the exocrine tissue. All vAChT labeled axons were also stained for synapsin (co-labeling appears yellow in F and inset G). Most synapsin labeled axons inside the islet did not contain vAChT (H). Scale bars = 20 μm (F) and 5 μm (in H, also applies to G). (I) Quantification of cholinergic axons (vAChT and synapsin double labeled axons) shows that their density is lower in the islet than in the exocrine tissue of the human pancreas (p < 0.01; Student’s t-test). Data are presented as mean ± SEM.
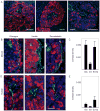
Figure 5. Endocrine cells in the mouse islet are more densely innervated by sympathetic, TH-labeled axons than endocrine cells in the human islet
(A) Human pancreatic sections immunostained for TH (green) and glucagon (red, left), insulin (red, middle), or somatostatin (red, right). Notice that TH labeled axons traverse the islet to reach regions devoid of endocrine cells. Scale bar = 50 μm. (B) Three maximal projections of confocal images showing immunostaining for TH (green) and glucagon (red, left), insulin (red, middle), or somatostatin (red, right) in mouse islets. TH-labeled axons localize to regions containing glucagon or somatostatin-labeled cells, but avoid regions containing insulin-labeled cells. Scale bar = 10 μm (applies to all panels). (C) Quantification of contacts between TH-labeled axons and endocrine cells shows that sympathetic axons preferentially target glucagon-labeled alpha (glu) and somatostatin-labeled delta cells (soma) in mouse islets. Contacts were automatically detected in three dimensions by Volocity software as “intersects” and were expressed as the ratio of the intersect volume to the volume of the respective endocrine cell immunostaining (contact density; see Experimental Procedures). The ratio is equivalent to the innervation density of each cell population. Ins = insulin. Data are presented as mean ± SEM. (D) Three maximal projections of confocal images showing immunostaining for TH (green) and glucagon (red, left), insulin (red, middle), or somatostatin (red, right) in human islets. TH-labeled axonal varicosities are present in regions devoid of endocrine cell immunostaining. (E) Quantification of contacts between TH-labeled axons and endocrine cells in human islets shows that, compared to mouse endocrine cells (B), human endocrine cells are sparsely innervated. Data are presented as mean ± SEM.
Figure 6. Blood vessels in human islets show a large proportion of smooth muscle cells
(A) Two maximal projections of confocal images showing immunostaining for TH (green) and the endothelial cell marker PECAM (red) in mouse (left) and human islets (right). In islets from both species, most TH-labeled axons run parallel to the trajectories of PECAM-labeled blood vessels. Islets are outlined with dashed line. Scale bars = 50 μm (left also applies to B, left; right also applies to B, right). (B) Two maximal projections of confocal images showing immunostaining for TH (green) and the smooth muscle cell marker alpha smooth muscle actin (SMA, red) in mouse (left) and human islets (right). Notice that blood vessels in the human islet contain many more SMA-labeled structures. TH-labeled fibers in human islets (arrows) seem to be exclusively associated with SMA-labeled regions. (C) Quantification shows a higher density of SMA-labeled smooth muscle cells in human islets (p < 0.05; Student’s t-test). Data are expressed as the ratio of detected SMA-labeled volume to the examined islet volume (see Experimental Procedures). Data are presented as mean ± SEM. (D) Maximal projection of confocal images of a human pancreatic section immunostained for alpha SMA (red) and PECAM (green). Notice that most vessels in the islet (dashed line) contain smooth alpha SMA-labeled vascular myocytes. Scale bar = 50 μm.
Figure 7. Sympathetic axons preferentially innervate smooth muscle cells in human islets
(A) TH-labeled axons (green) are localized to regions containing SMA-labeled smooth muscle cells (red) in human islets. Scale bar = 50 μm. (B) Quantification of cell contacts shows that TH-labeled axons preferentially contact smooth muscle cells inside the islet (SMA; filled bars) as well as in the exocrine tissue (empty bars). Few contacts with endocrine cells (Glu and Ins) and ductal cells (CK 19) could be detected. Contacts were expressed as the ratio of the “intersect” volume over the volume of the respective cell immunostaining (contact density; see Experimental Procedures). The ratio is equivalent to the innervation density of each cell population. Data are presented as mean ± SEM. (C–D) TH labeled axons and varicosities were seen contouring blood vessels in the exocrine tissue, where they are close to SMA-labled smooth muscle cells (C) and endothelial cells (D). Th-labeled axons can be also seen in the vicinity of CK 19-labeled ductal cells (E).
Comment in
- Islets have a lot of nerve! Or do they?
Taborsky GJ Jr. Taborsky GJ Jr. Cell Metab. 2011 Jul 6;14(1):5-6. doi: 10.1016/j.cmet.2011.06.004. Cell Metab. 2011. PMID: 21723498 Free PMC article. - Highlights in basic autonomic neurosciences: autonomic control of the counter-regulatory response and glucose homeostasis.
Verberne AJ, Gilbey MP. Verberne AJ, et al. Auton Neurosci. 2012 Jul 2;169(1):1-3. doi: 10.1016/j.autneu.2012.04.002. Epub 2012 May 9. Auton Neurosci. 2012. PMID: 22578333 No abstract available.
Similar articles
- Novel approaches to studying the role of innervation in the biology of pancreatic islets.
Rodriguez-Diaz R, Caicedo A. Rodriguez-Diaz R, et al. Endocrinol Metab Clin North Am. 2013 Mar;42(1):39-56. doi: 10.1016/j.ecl.2012.11.001. Epub 2012 Dec 20. Endocrinol Metab Clin North Am. 2013. PMID: 23391238 Free PMC article. Review. - Neural control of the endocrine pancreas.
Rodriguez-Diaz R, Caicedo A. Rodriguez-Diaz R, et al. Best Pract Res Clin Endocrinol Metab. 2014 Oct;28(5):745-56. doi: 10.1016/j.beem.2014.05.002. Epub 2014 May 20. Best Pract Res Clin Endocrinol Metab. 2014. PMID: 25256769 Review. - Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function.
Rodriguez-Diaz R, Speier S, Molano RD, Formoso A, Gans I, Abdulreda MH, Cabrera O, Molina J, Fachado A, Ricordi C, Leibiger I, Pileggi A, Berggren PO, Caicedo A. Rodriguez-Diaz R, et al. Proc Natl Acad Sci U S A. 2012 Dec 26;109(52):21456-61. doi: 10.1073/pnas.1211659110. Epub 2012 Dec 10. Proc Natl Acad Sci U S A. 2012. PMID: 23236142 Free PMC article. - 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury.
Chiu YC, Hua TE, Fu YY, Pasricha PJ, Tang SC. Chiu YC, et al. Diabetologia. 2012 Dec;55(12):3252-61. doi: 10.1007/s00125-012-2699-6. Epub 2012 Aug 30. Diabetologia. 2012. PMID: 22930160 - Parasympathetic innervation and function of endocrine pancreas requires the glial cell line-derived factor family receptor alpha2 (GFRalpha2).
Rossi J, Santamäki P, Airaksinen MS, Herzig KH. Rossi J, et al. Diabetes. 2005 May;54(5):1324-30. doi: 10.2337/diabetes.54.5.1324. Diabetes. 2005. PMID: 15855316
Cited by
- Pancreatic islet blood flow and its measurement.
Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, Grapensparr L, Källskog Ö, Lau J, Liljebäck H, Palm F, Quach M, Sandberg M, Strömberg V, Ullsten S, Carlsson PO. Jansson L, et al. Ups J Med Sci. 2016 May;121(2):81-95. doi: 10.3109/03009734.2016.1164769. Epub 2016 Apr 28. Ups J Med Sci. 2016. PMID: 27124642 Free PMC article. Review. - Structural similarities and differences between the human and the mouse pancreas.
Dolenšek J, Rupnik MS, Stožer A. Dolenšek J, et al. Islets. 2015;7(1):e1024405. doi: 10.1080/19382014.2015.1024405. Islets. 2015. PMID: 26030186 Free PMC article. Review. - Islet nerves in focus--defining their neurobiological and clinical role.
Ahrén B. Ahrén B. Diabetologia. 2012 Dec;55(12):3152-4. doi: 10.1007/s00125-012-2727-6. Epub 2012 Sep 22. Diabetologia. 2012. PMID: 23001378 - Novel approaches to studying the role of innervation in the biology of pancreatic islets.
Rodriguez-Diaz R, Caicedo A. Rodriguez-Diaz R, et al. Endocrinol Metab Clin North Am. 2013 Mar;42(1):39-56. doi: 10.1016/j.ecl.2012.11.001. Epub 2012 Dec 20. Endocrinol Metab Clin North Am. 2013. PMID: 23391238 Free PMC article. Review. - Parallel Control of an Artificial Pancreas with Coordinated Insulin, Glucagon, and Rescue Carbohydrate Control Actions.
Moscardó V, Díez JL, Bondia J. Moscardó V, et al. J Diabetes Sci Technol. 2019 Nov;13(6):1026-1034. doi: 10.1177/1932296819879093. Epub 2019 Oct 20. J Diabetes Sci Technol. 2019. PMID: 31631688 Free PMC article.
References
- Ahrén B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. - PubMed
- Ahrén B, Ar’Rajab A, Böttcher G, Sundler F, Dunning B. Presence of galanin in human pancreatic nerves and inhibition of insulin secretion from isolated human islets. Cell Tissue Res. 1991;264:263–267. - PubMed
- Ahrén B, Holst J. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50:1030–1038. - PubMed
- Ahrén B, Taborsky GJ, Porte DJ. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1986;29:827–836. - PubMed
- Ahrén B, Veith R, Taborsky GJ. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology. 1987;121:323–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK084321-01A1/DK/NIDDK NIH HHS/United States
- F32 DK083226-01A1/DK/NIDDK NIH HHS/United States
- R56 DK084321/DK/NIDDK NIH HHS/United States
- F32 DK083226-03/DK/NIDDK NIH HHS/United States
- F32 DK083226/DK/NIDDK NIH HHS/United States
- R01 DK084321/DK/NIDDK NIH HHS/United States
- F32DK083226/DK/NIDDK NIH HHS/United States
- R01DK084321/DK/NIDDK NIH HHS/United States
- 5U01DK070460-07/DK/NIDDK NIH HHS/United States
- F32 DK083226-02/DK/NIDDK NIH HHS/United States
- R56 DK084321-01/DK/NIDDK NIH HHS/United States
- R56DK084321/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources