Complex inhibitory effects of nitric oxide on autophagy - PubMed (original) (raw)
. 2011 Jul 8;43(1):19-32.
doi: 10.1016/j.molcel.2011.04.029.
Viktor I Korolchuk, Maurizio Renna, Sara Imarisio, Angeleen Fleming, Andrea Williams, Moises Garcia-Arencibia, Claudia Rose, Shouqing Luo, Benjamin R Underwood, Guido Kroemer, Cahir J O'Kane, David C Rubinsztein
Affiliations
- PMID: 21726807
- PMCID: PMC3149661
- DOI: 10.1016/j.molcel.2011.04.029
Complex inhibitory effects of nitric oxide on autophagy
Sovan Sarkar et al. Mol Cell. 2011.
Abstract
Autophagy, a major degradation process for long-lived and aggregate-prone proteins, affects various human processes, such as development, immunity, cancer, and neurodegeneration. Several autophagy regulators have been identified in recent years. Here we show that nitric oxide (NO), a potent cellular messenger, inhibits autophagosome synthesis via a number of mechanisms. NO impairs autophagy by inhibiting the activity of S-nitrosylation substrates, JNK1 and IKKβ. Inhibition of JNK1 by NO reduces Bcl-2 phosphorylation and increases the Bcl-2-Beclin 1 interaction, thereby disrupting hVps34/Beclin 1 complex formation. Additionally, NO inhibits IKKβ and reduces AMPK phosphorylation, leading to mTORC1 activation via TSC2. Overexpression of nNOS, iNOS, or eNOS impairs autophagosome formation primarily via the JNK1-Bcl-2 pathway. Conversely, NOS inhibition enhances the clearance of autophagic substrates and reduces neurodegeneration in models of Huntington's disease. Our data suggest that nitrosative stress-mediated protein aggregation in neurodegenerative diseases may be, in part, due to autophagy inhibition.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
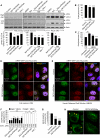
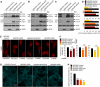
NO Inhibits Autophagosome Synthesis (A) Immunoblot analysis with anti-LC3 antibody shows that NO donors reduced LC3-II levels in rat primary cortical neurons and decreased autophagosome synthesis in bafilomycin A1-treated rat primary cortical neurons and HeLa cells. (B–E) Confocal microscopy images and analysis of autophagic flux by automated Cellomics microscopy show that NO donors reduced autolysosome numbers in mRFP-GFP-LC3 HeLa cells grown in full medium (FM) (B and D) or starved with HBSS (C and E). Cells in HBSS had more autolysosomes compared to FM (C). Autolysosome number in untreated cells in FM is set at 100%. (F) NO donors increased EGFP-HDQ74 aggregates in a dose-dependent manner in _EGFP-HDQ74_–transfected Atg5+/+, but not in _Atg5_−/−, MEFs. _Atg5_−/− MEFs had more EGFP-HDQ74 aggregates than Atg5+/+ MEFs. (G and H) Confocal microscopy images (H) and immunofluorescence analysis with anti-Atg16 antibody (G) show that NO donors reduced the percentage of HeLa cells with Atg16-positive structures (arrows) under starvation. Graphical data denote mean ± SEM.
Figure 2
NO Reduced Phosphorylation of JNK1 and Bcl-2, Leading to Increased Bcl-2–Beclin 1 and Decreased Beclin 1–hVps34 Interactions (A) NO donors reduced JNK1 phosphorylation in HeLa cells, as analyzed by immunoprecipitation with anti-JNK1 agarose-conjugated beads and immunoblotting with anti-phospho-JNK antibody. (B) NO donors reduced Bcl-2 phosphorylation in HeLa cells, as analyzed by immunoblotting with anti-phospho-Bcl-2 antibody. (C) Immunoblot analysis with anti-phospho-Bcl-2 antibody shows that DETA NONOate could not reduce phospho-Bcl-2 in _Flag-CA JNK1_-transfected HeLa cells, where phospho-Bcl-2 was higher compared to mock-transfected cells. (D and E) Immunoprecipitation with anti-Flag M2 affinity agarose gel and immunoblotting with anti-Myc antibody shows that NO donors increased the interaction of Flag-Beclin 1 with WT Myc-Bcl-2 (D), but not with AAA Myc-Bcl-2 (E), in HeLa cells transfected with WT Myc-Bcl-2 (D) or AAA Myc-Bcl-2 (E) along with either empty Flag or Flag-Beclin 1. Asterisk denotes IgG band (E). (F) Immunoprecipitation with anti-Flag M2 affinity agarose gel and immunoblotting with anti-Vps34 antibody shows that NO donors decreased Flag-Beclin 1–hVps34 interaction in HeLa cells transfected with hVps34 along with either empty Flag or Flag-Beclin 1. (G) Immunoprecipitation with anti-Flag M2 affinity agarose gel and immunoblotting with anti-Vps34 antibody shows that AAA Myc-Bcl-2 decreased Flag-Beclin 1–hVps34 interaction in the presence (right panel) or absence (left panel) of DETA NONOate in HeLa cells transfected with hVps34 along with either empty Flag or Flag-Beclin 1, and with pcDNA3.1 or AAA Myc-Bcl-2. Graphical data denote mean ± SEM.
Figure 3
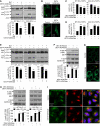
NO Impairs Autophagy in AAA Bcl-2 and _Jnk1_−/− MEFs by Activating mTORC1 (A) Immunoblot analysis with anti-LC3 antibody shows that DETA NONOate reduced autophagosome synthesis in bafilomycin A1-treated WT Bcl-2 and AAA Bcl-2 MEFs. (B and C) NO donors decreased EGFP-LC3 vesicles in _EGFP-LC3_–transfected WT Bcl-2 and AAA Bcl-2 MEFs (C). Images were acquired by a confocal microscope (B). WT Bcl-2 and AAA Bcl-2 MEFs were analyzed separately. (D) NO donors increased EGFP-HDQ74 aggregates in _EGFP-HDQ74_–transfected WT Bcl-2 and AAA Bcl-2 MEFs. WT Bcl-2 and AAA Bcl-2 MEFs were analyzed separately. (E) Immunoblot analysis with anti-LC3 antibody shows that DETA NONOate reduced autophagosome synthesis in bafilomycin A1-treated WT Jnk, _Jnk1_−/−, and _Jnk2_−/− MEFs. (F) Immunoblot analyses with anti-phospho-S6K and anti-phospho-S6 antibodies show that NO donors activated mTORC1 in HeLa cells. (G) Confocal microscope images of immunofluorescence with anti-phospho-S6 antibody show that DETA NONOate increased S6 phosphorylation; arrowhead shows a cell where this effect was not observed. (H) Immunoblot analyses with anti-phospho-S6K and anti-phospho-S6 antibodies show that NO donors activated mTORC1 in WT Bcl-2 and AAA Bcl-2 MEFs. (I) Confocal microscope images of immunofluorescence with anti-phospho-mTOR and anti-LAMP1 antibodies in HeLa cells show that DETA NONOate increased phospho-mTOR but did not alter its distribution with lysosomes. Graphical data denote mean ± SEM.
Figure 4
Activation of mTORC1 by NO Is Dependent on TSC2 and IKKβ (A) Immunoblot analyses with anti-phospho-S6K and anti-phospho-S6 antibodies show that NO donors activated mTORC1 in Tsc2+/+ MEFs, but not in _Tsc2_−/−, MEFs. (B) NO donors reduced phosphorylation of IKKα/β and AMPKα in MEFs, as analyzed by immunoblotting with anti-phospho-IKKα/β and anti-phospho-AMPKα antibodies, respectively. (C) Immunoblot analyses with anti-phospho-S6K and anti-phospho-S6 antibodies show that NO donors activated mTORC1 in Ikkβ+/+ MEFs, but not in _Ikkβ_−/− MEFs. (D) Immunoblot analysis with anti-LC3 antibody shows that DETA NONOate reduced autophagosome synthesis in bafilomycin A1-treated Tsc2+/+ and _Tsc2_−/− MEFs. (E) DETA NONOate decreased EGFP-LC3 vesicles in Tsc2+/+ and _Tsc2_−/− MEFs transfected with EGFP-LC3, along with either pcDNA3.1 or AAA Myc-Bcl-2. Although it further reduced EGFP-LC3 vesicles in Tsc2+/+ MEFs expressing AAA Myc-Bcl-2 compared to mock-transfected Tsc2+/+ MEFs, this effect was not seen in _Tsc2_−/− MEFs. Tsc2+/+ and _Tsc2_−/− MEFs were analyzed separately. Graphical data denote mean ± SEM.
Figure 5
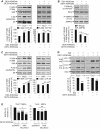
Overexpression of Mammalian NOS Isoforms Inhibits Autophagosome Synthesis (A–D) Immunoblot analyses with anti-LC3, along with anti-nNOS, anti-iNOS, and anti-eNOS antibodies, show decreased LC3-II levels in stable HEK293 cell lines overexpressing nNOS (A and D), iNOS-GFP (B and D), or eNOS (C and D) compared to HEK293 control cells in the presence or absence of bafilomycin A1. (E and F) mCherry-LC3 vesicles were reduced in _mCherry-LC3–_transfected stable HEK293 NOS cell lines compared to HEK293 control cells cultured in HBSS, an effect that was restored in the NOS cells by L-NAME (F). Images were acquired by a confocal microscope (E). (G and H) Confocal microscopy images (G) and immunofluorescence analysis with anti-Atg16 antibody show that stable HEK293 NOS cell lines had fewer Atg16-positive structures under starvation, compared to HEK293 control cells (H). Graphical data denote mean ± SEM.
Figure 6
NOS Overexpression Primarily Inhibits the JNK1–Bcl-2 Pathway to Impair Autophagy (A–F) Immunoblot analyses with anti-nNOS, anti-iNOS, anti-eNOS, anti-phospho-JNK1, anti-phospho-Bcl-2, anti-phospho-S6K, and anti-phospho-S6 antibodies show that stable HEK293 cell lines overexpressing nNOS (A and D), iNOS-GFP (B and E), or eNOS (C and F) inhibited both the JNK1–Bcl-2 and mTORC1 pathways compared to the HEK293 control cells under basal (FM) (A–C) or starvation (HBSS) (D–F) conditions. Graphical data denote mean ± SEM.
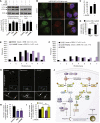
Figure 7
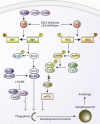
L-NAME Induces Autophagy and Reduces Mutant Huntingtin Aggregation/Neurodegeneration in Models of Huntington's Disease (A) Immunoblot analyses with anti-LC3 antibody show that rapamycin (8 hr) and L-NAME (24 hr) increased autophagosome synthesis in bafilomycin A1-treated mouse primary cortical neurons and HeLa cells. (B and C) Confocal microscopy images (B) and analysis of autophagic flux by automated Cellomics microscope (C) show that L-NAME increased autolysosomes in mRFP-GFP-LC3 HeLa cells. (D) L-NAME reduced EGFP-HDQ74 aggregates in _EGFP-HDQ74_–transfected Atg5+/+, but not in _Atg5_−/−, MEFs. (E) Drosophila expressing mutant huntingtin exon 1 (Q120) shows a significant decrease in neurodegeneration (p < 0.001, paired t test) upon L-NAME treatment compared to DMSO. (F) Expression of an endogenous negative regulator of NOS activity (NOS4) significantly attenuates neurodegeneration (p < 0.05, paired t test) in Drosophila expressing mutant huntingtin exon 1 (Q120). (G–I) Images from the retina of transgenic HD zebrafish show mutant huntingtin aggregates (arrows) (G). Treatment with rapamycin or L-NAME reduced the number of aggregates (H). L-NAME did not reduce aggregates in the presence of NH4Cl, which increased aggregate count (I). (J) NO inhibits autophagy by _S_-nitrosylating and inhibiting JNK1 phosphorylation, thereby reducing phospho-Bcl-2 and increasing Bcl-2–Beclin 1 interaction, which disrupts hVps34–Beclin 1 association. NO also _S_-nitrosylates and inhibits IKKβ phosphorylation, leading to reduced phospho-AMPK and TSC2 activity, which alleviates the inhibitory effect of TSC1/2 on Rheb (denoted by “×”), thereby allowing Rheb to activate mTORC1 and inhibit autophagy. Overexpression of NOS isoforms impairs autophagy by inhibiting the JNK1 pathway, whereas NOS inhibition by L-NAME induces autophagy by mechanism distinct from the NO pathways. Graphical data denote mean ± SEM, except in (E) and (F).
Comment in
- S-Nitrosylation at the interface of autophagy and disease.
Haldar SM, Stamler JS. Haldar SM, et al. Mol Cell. 2011 Jul 8;43(1):1-3. doi: 10.1016/j.molcel.2011.06.014. Mol Cell. 2011. PMID: 21726803
Similar articles
- Rhes, a striatal-selective protein implicated in Huntington disease, binds beclin-1 and activates autophagy.
Mealer RG, Murray AJ, Shahani N, Subramaniam S, Snyder SH. Mealer RG, et al. J Biol Chem. 2014 Feb 7;289(6):3547-54. doi: 10.1074/jbc.M113.536912. Epub 2013 Dec 9. J Biol Chem. 2014. PMID: 24324270 Free PMC article. - The GST-BHMT assay reveals a distinct mechanism underlying proteasome inhibition-induced macroautophagy in mammalian cells.
Rui YN, Xu Z, Chen Z, Zhang S. Rui YN, et al. Autophagy. 2015;11(5):812-32. doi: 10.1080/15548627.2015.1034402. Autophagy. 2015. PMID: 25984893 Free PMC article. - JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy.
Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. Wei Y, et al. Mol Cell. 2008 Jun 20;30(6):678-88. doi: 10.1016/j.molcel.2008.06.001. Mol Cell. 2008. PMID: 18570871 Free PMC article. - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - The Function of Autophagy in Neurodegenerative Diseases.
Kiriyama Y, Nochi H. Kiriyama Y, et al. Int J Mol Sci. 2015 Nov 9;16(11):26797-812. doi: 10.3390/ijms161125990. Int J Mol Sci. 2015. PMID: 26569220 Free PMC article. Review.
Cited by
- αSynuclein and Mitochondrial Dysfunction: A Pathogenic Partnership in Parkinson's Disease?
Protter D, Lang C, Cooper AA. Protter D, et al. Parkinsons Dis. 2012;2012:829207. doi: 10.1155/2012/829207. Epub 2012 Jun 10. Parkinsons Dis. 2012. PMID: 22737587 Free PMC article. - mTOR-Independent autophagy inducer trehalose rescues against insulin resistance-induced myocardial contractile anomalies: Role of p38 MAPK and Foxo1.
Wang Q, Ren J. Wang Q, et al. Pharmacol Res. 2016 Sep;111:357-373. doi: 10.1016/j.phrs.2016.06.024. Epub 2016 Jun 27. Pharmacol Res. 2016. PMID: 27363949 Free PMC article. - Oral Carbon Monoxide Enhances Autophagy Modulation in Prostate, Pancreatic, and Lung Cancers.
Bi J, Witt E, McGovern MK, Cafi AB, Rosenstock LL, Pearson AB, Brown TJ, Karasic TB, Absler LC, Machkanti S, Boyce H, Gallo D, Becker SL, Ishida K, Jenkins J, Hayward A, Scheiflinger A, Bodeker KL, Kumar R, Shaw SK, Jabbour SK, Lira VA, Henry MD, Tift MS, Otterbein LE, Traverso G, Byrne JD. Bi J, et al. Adv Sci (Weinh). 2024 Mar;11(9):e2308346. doi: 10.1002/advs.202308346. Epub 2023 Dec 12. Adv Sci (Weinh). 2024. PMID: 38084435 Free PMC article. - Context-Dependent Regulation of Autophagy by IKK-NF-κB Signaling: Impact on the Aging Process.
Salminen A, Hyttinen JM, Kauppinen A, Kaarniranta K. Salminen A, et al. Int J Cell Biol. 2012;2012:849541. doi: 10.1155/2012/849541. Epub 2012 Jul 26. Int J Cell Biol. 2012. PMID: 22899934 Free PMC article. - Burkholderia pseudomallei rpoS mediates iNOS suppression in human hepatocyte (HC04) cells.
Sanongkiet S, Ponnikorn S, Udomsangpetch R, Tungpradabkul S. Sanongkiet S, et al. FEMS Microbiol Lett. 2016 Aug;363(15):fnw161. doi: 10.1093/femsle/fnw161. Epub 2016 Jun 19. FEMS Microbiol Lett. 2016. PMID: 27324398 Free PMC article.
References
- Azad N., Vallyathan V., Wang L., Tantishaiyakul V., Stehlik C., Leonard S.S., Rojanasakul Y. S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J. Biol. Chem. 2006;281:34124–34134. - PubMed
- Chen Y., Azad M.B., Gibson S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. - PubMed
- Denninger J.W., Marletta M.A. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. Biophys. Acta. 1999;1411:334–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous