Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes - PubMed (original) (raw)
Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes
Ikwunga Wonodi et al. Arch Gen Psychiatry. 2011 Jul.
Abstract
Context: Kynurenic acid, a metabolite of the kynurenine pathway of tryptophan degradation, is an antagonist at N-methyl-d-aspartate and α7 nicotinic acetylcholine receptors and modulates glutamate, dopamine, and acetylcholine signaling. Cortical kynurenic acid concentrations are elevated in the brain and cerebrospinal fluid of schizophrenia patients. The proximal cause may be an impairment of kynurenine 3-monooxygenase (KMO), a rate-limiting enzyme at the branching point of the kynurenine pathway.
Objectives: To examine KMO messenger RNA expression and KMO enzyme activity in postmortem tissue from the frontal eye field (FEF; Brodmann area 6) obtained from schizophrenia individuals compared with healthy control individuals and to explore the relationship between KMO single-nucleotide polymorphisms and schizophrenia oculomotor endophenotypes.
Design: Case-control postmortem and clinical study.
Setting: Maryland Brain Collection, outpatient clinics.
Participants: Postmortem specimens from schizophrenia patients (n = 32) and control donors (n = 32) and a clinical sample of schizophrenia patients (n = 248) and healthy controls (n = 228).
Main outcome measures: Comparison of quantitative KMO messenger RNA expression and KMO enzyme activity in postmortem FEF tissue between schizophrenia patients and controls and association of KMO single-nucleotide polymorphisms with messenger RNA expression in postmortem FEF and schizophrenia and oculomotor endophenotypes (ie, smooth pursuit eye movements and oculomotor delayed response).
Results: In postmortem tissue, we found a significant and correlated reduction in KMO gene expression and KMO enzyme activity in the FEF in schizophrenia patients. In the clinical sample, KMO rs2275163 was not associated with a diagnosis of schizophrenia but showed modest effects on predictive pursuit and visuospatial working memory endophenotypes.
Conclusion: Our results provide converging lines of evidence implicating reduced KMO activity in the etiopathophysiology of schizophrenia and related neurocognitive deficits.
Figures
Figure 1
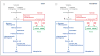
The kynurenine pathway of tryptophan degradation. A, Metabolism is initiated by the oxidative ring opening of tryptophan by indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase. In the brain, the pivotal metabolite kynurenine is enzymatically converted to 3-hydroxykynurenine and kynurenic acid in microglial cells and astrocytes, respectively. B, In schizophrenia, a persistent reduction of microglial kynurenine 3-monooxygenase activity would result in increased kynurenic acid formation in, and release from, astrocytes. This could cause increased inhibition of neuronal α7 nicotinic receptors (α7nAChRs) and _N_-methyl-
d
-aspartate receptors (NMDARs) (modified from Wonodi and Schwarcz). NAD+ indicates nicotinamide adenine dinucleotide.
Figure 2
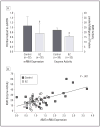
KMO messenger RNA (mRNA) expression and kynurenine 3-monooxygenase (KMO) enzyme activity. A, Mean KMO mRNA expression and KMO enzyme activity in frontal eye field (FEF) tissues obtained post mortem from schizophrenia (SZ) patients and healthy control individuals. Both measures are significantly reduced in the FEF of SZ patients: *P<.05, †_P_=.005 (analysis of variance). Error bars indicate SD; RQ, relative quantitation. B, Scatterplot of Pearson correlation (_r_= 0.66) between KMO gene expression (RQ values, normalized to GAPDH) and KMO enzyme activity in FEF tissue from 30 healthy controls and 30 SZ patients.
Figure 3
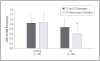
Mean effects of kynurenine 3-monooxygenase (KMO) rs2275163 genotype groups on KMO gene expression (ie, relative quantitation values normalized to GAPDH) in postmortem frontal eye field (FEF) samples. KMO gene expression is compared between carriers of the minor allele (TT and CT) (black bars) and carriers that are homozygous for the major allele (CC) (gray bars) in control and schizophrenia FEF tissue specimens: *P<.05, nominal (analysis of variance). Error bars indicate SD. TT and CT: control specimens: n=18, schizophrenia specimens: n=17; CC: control specimens: n=14, schizophrenia specimens: n=15.
Figure 4
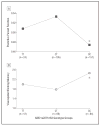
Results of single-nucleotide polymorphism (SNP) genotyping. A, Effect of kynurenine 3-monooxygenase (KMO) SNP rs2275163 CC genotype on predictive pursuit function in the combined clinical sample of schizophrenia patients and healthy control individuals. Participants with the CC genotype had significantly worse predictive pursuit function compared with participants with CT or TT genotypes: *P<.003 (analysis of variance post hoc test). B, Effect of KMO SNP rs2275163 CC genotype on visuospatial working memory in the combined clinical sample of schizophrenia patients and healthy control participants. Participants with the CC genotype made significantly more visuospatial (spatial) working memory errors compared with participants with the CT or TT genotypes: *P<.009 (analysis of variance post hoc test).
Similar articles
- Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls.
Holtze M, Saetre P, Engberg G, Schwieler L, Werge T, Andreassen OA, Hall H, Terenius L, Agartz I, Jönsson EG, Schalling M, Erhardt S. Holtze M, et al. J Psychiatry Neurosci. 2012 Jan;37(1):53-7. doi: 10.1503/jpn.100175. J Psychiatry Neurosci. 2012. PMID: 21693093 Free PMC article. - Influence of kynurenine 3-monooxygenase (KMO) gene polymorphism on cognitive function in schizophrenia.
Wonodi I, McMahon RP, Krishna N, Mitchell BD, Liu J, Glassman M, Hong LE, Gold JM. Wonodi I, et al. Schizophr Res. 2014 Dec;160(1-3):80-7. doi: 10.1016/j.schres.2014.10.026. Epub 2014 Nov 14. Schizophr Res. 2014. PMID: 25464917 Free PMC article. - Maternal genotype determines kynurenic acid levels in the fetal brain: Implications for the pathophysiology of schizophrenia.
Beggiato S, Notarangelo FM, Sathyasaikumar KV, Giorgini F, Schwarcz R. Beggiato S, et al. J Psychopharmacol. 2018 Nov;32(11):1223-1232. doi: 10.1177/0269881118805492. Epub 2018 Oct 24. J Psychopharmacol. 2018. PMID: 30354938 - Kynurenine-3-monooxygenase (KMO): From its biological functions to therapeutic effect in diseases progression.
Chen Y, Zhang J, Yang Y, Xiang K, Li H, Sun D, Chen L. Chen Y, et al. J Cell Physiol. 2022 Dec;237(12):4339-4355. doi: 10.1002/jcp.30876. Epub 2022 Sep 11. J Cell Physiol. 2022. PMID: 36088660 Review. - The kynurenine pathway in schizophrenia and bipolar disorder.
Erhardt S, Schwieler L, Imbeault S, Engberg G. Erhardt S, et al. Neuropharmacology. 2017 Jan;112(Pt B):297-306. doi: 10.1016/j.neuropharm.2016.05.020. Epub 2016 May 28. Neuropharmacology. 2017. PMID: 27245499 Review.
Cited by
- Associations of the serum kynurenine pathway metabolites with P50 auditory gating in non-smoking patients with first-episode schizophrenia.
Yang Q, Zhang Y, Yang K, Niu Y, Fan F, Chen S, Luo X, Tan S, Wang Z, Tong J, Yang F, Li CR, Tan Y. Yang Q, et al. Front Psychiatry. 2022 Oct 20;13:1036421. doi: 10.3389/fpsyt.2022.1036421. eCollection 2022. Front Psychiatry. 2022. PMID: 36339840 Free PMC article. - Changes in kynurenine metabolites in the gray and white matter of the dorsolateral prefrontal cortex of individuals affected by schizophrenia.
Antenucci N, D'Errico G, Fazio F, Nicoletti F, Bruno V, Battaglia G. Antenucci N, et al. Schizophrenia (Heidelb). 2024 Feb 27;10(1):27. doi: 10.1038/s41537-024-00447-3. Schizophrenia (Heidelb). 2024. PMID: 38413629 Free PMC article. - Pharmacophore-Based Virtual Screening of Novel Competitive Inhibitors of the Neurodegenerative Disease Target Kynurenine-3-Monooxygenase.
Gotina L, Seo SH, Kim CW, Lim SM, Pae AN. Gotina L, et al. Molecules. 2021 May 31;26(11):3314. doi: 10.3390/molecules26113314. Molecules. 2021. PMID: 34073016 Free PMC article. - Multiomic profiling of iron-deficient infant monkeys reveals alterations in neurologically important biochemicals in serum and cerebrospinal fluid before the onset of anemia.
Sandri BJ, Kim J, Lubach GR, Lock EF, Guerrero C, Higgins L, Markowski TW, Kling PJ, Georgieff MK, Coe CL, Rao RB. Sandri BJ, et al. Am J Physiol Regul Integr Comp Physiol. 2022 Jun 1;322(6):R486-R500. doi: 10.1152/ajpregu.00235.2021. Epub 2022 Mar 10. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 35271351 Free PMC article. - Importance of kynurenine 3-monooxygenase for spontaneous firing and pharmacological responses of midbrain dopamine neurons: Relevance for schizophrenia.
Tufvesson-Alm M, Schwieler L, Schwarcz R, Goiny M, Erhardt S, Engberg G. Tufvesson-Alm M, et al. Neuropharmacology. 2018 Aug;138:130-139. doi: 10.1016/j.neuropharm.2018.06.003. Epub 2018 Jun 5. Neuropharmacology. 2018. PMID: 29879409 Free PMC article.
References
- Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50(7):521–530. - PubMed
- Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol Behav. 2007;92(1-2):203–209. - PubMed
- Müller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10(2):131–148. - PubMed
- Miller CL, Llenos IC, Cwik M, Walkup J, Weis S. Alterations in kynurenine precursor and product levels in schizophrenia and bipolar disorder. Neurochem Int. 2008;52(6):1297–1303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01RR16500/RR/NCRR NIH HHS/United States
- K12 RR023250/RR/NCRR NIH HHS/United States
- R01 MH067014/MH/NIMH NIH HHS/United States
- MH67014/MH/NIMH NIH HHS/United States
- MH079172/MH/NIMH NIH HHS/United States
- M01 RR016500/RR/NCRR NIH HHS/United States
- R01 MH049826/MH/NIMH NIH HHS/United States
- MH68580/MH/NIMH NIH HHS/United States
- MH075101/MH/NIMH NIH HHS/United States
- MH-K12RR023250/MH/NIMH NIH HHS/United States
- R01 DA027680/DA/NIDA NIH HHS/United States
- P30 MH068580/MH/NIMH NIH HHS/United States
- R03 MH075101/MH/NIMH NIH HHS/United States
- R21 MH079172/MH/NIMH NIH HHS/United States
- R01 MH085646/MH/NIMH NIH HHS/United States
- MH49826/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical