Human mediator subunit MED26 functions as a docking site for transcription elongation factors - PubMed (original) (raw)
. 2011 Jul 8;146(1):92-104.
doi: 10.1016/j.cell.2011.06.005.
Tari J Parmely, Shigeo Sato, Chieri Tomomori-Sato, Charles A S Banks, Stephanie E Kong, Henrietta Szutorisz, Selene K Swanson, Skylar Martin-Brown, Michael P Washburn, Laurence Florens, Chris W Seidel, Chengqi Lin, Edwin R Smith, Ali Shilatifard, Ronald C Conaway, Joan W Conaway
Affiliations
- PMID: 21729782
- PMCID: PMC3145325
- DOI: 10.1016/j.cell.2011.06.005
Human mediator subunit MED26 functions as a docking site for transcription elongation factors
Hidehisa Takahashi et al. Cell. 2011.
Abstract
Promoter-proximal pausing by initiated RNA polymerase II (Pol II) and regulated release of paused polymerase into productive elongation has emerged as a major mechanism of transcription activation. Reactivation of paused Pol II correlates with recruitment of super-elongation complexes (SECs) containing ELL/EAF family members, P-TEFb, and other proteins, but the mechanism of their recruitment is an unanswered question. Here, we present evidence for a role of human Mediator subunit MED26 in this process. We identify in the conserved N-terminal domain of MED26 overlapping docking sites for SEC and a second ELL/EAF-containing complex, as well as general initiation factor TFIID. In addition, we present evidence consistent with the model that MED26 can function as a molecular switch that interacts first with TFIID in the Pol II initiation complex and then exchanges TFIID for complexes containing ELL/EAF and P-TEFb to facilitate transition of Pol II into the elongation stage of transcription.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
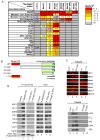
Figure 1. Association of Med26 NTD with transcription elongation factors, MLL fusion partners, and TFIID
(A) MudPIT of Med26-associated proteins. See also Table S1. (B) Schematic of wild type and mutant Med26 proteins. (C) Western blot of FLAG-IPed complexes from HeLa cells expressing wild type and mutant Med26 (lanes 2-5, 7-10) and of rat liver Pol II (lane 1) (Serizawa et al., 1992). Lanes 1-3 and 4-5, respectively, are from different blots. (D) Proteins copurifying with FLAG-Med26 NTD, TFIIS NTD, or Elongin A NTD, analyzed by western blotting. WT, wild type. Here and in subsequent figures, IP and input (3% of total) were analyzed on different blots; hence it is not possible to make quantitative comparisons between IP and input samples.
Figure 2. ELL/EAF-containing complexes bind the Med26 NTD
(A) MudPIT of proteins bound to SEC components, NARG2, and KIAA0947. (B) Western blotting of proteins associated with FLAG-tagged NARG2, KIAA0947 and AF9. (C) Binding of NARG2 and KIAA0947 to ELL/EAF1. FLAG-IPed complexes from baculovirus-infected insect cells expressing the indicated proteins were analyzed by western blotting. Asterisk, IgH. (D) Binding of EAF1 to the Med26 NTD. Western blotting of FLAG-IPed complexes or lysates (Input) from baculovirus-infected insect cells, using anti-FLAG (M2) antibodies and Alexa Fluor 680-labeled anti-mouse IgG (light chain-specific) (red) or rabbit anti-cMyc antibodies and IR Dye™ 800-labeled goat anti rabbit IgG (green).
Figure 3. The Med26 NTD is needed for Mediator-dependent recruitment of ELL/EAF but not TFIID to promoters
(A) Schematic of immobilized template assay. (B) Med26 NTD is dispensable for Mediator-dependent recruitment of TFIID to a promoter. Assays contained GAL4×5-MLT template and GAL4-VP16, TFIID, and Mediator (Med) with Med26 or Med26ΔNTD as indicated. (C) Mediator-dependent recruitment of ELL/EAF1 to GAL4- (lanes 1-3) or HNF4α-responsive promoters. Med(F-Med26 wt), Med26-Mediator. (D) Med26ΔNTD-Mediator [Med(F-Med26 ΔNTD)] does not recruit EAF1 to a promoter. (E) Free Med26 is not sufficient for EAF1 recruitment. Lanes 1-4, assays contained either free full length Med26 or Med26-Mediator. Lanes 5-8 show amounts of free Med26 and Med26-Mediator in binding assays. (F) Free Med26 NTD blocks Mediator-dependent EAF1 recruitment. Assays were performed with Med26-Mediator, with or without free Med26 NTD (His-Med26-NTD).
Figure 4. Med26 NTD mutants fail to bind ELL/EAF-containing complexes or TFIID
(A) Western blots of FLAG IPs or lysates (Input) from cells stably expressing the indicated FLAG-Med26 NTD proteins. Data is from the same experiment in Figure 1D. Negative and positive controls in the first two lanes of each panel are repeated from Figure 1D. (B) Defective binding of Med26(R61A,K62A)-Mediator to elongation factors. FLAG IPs from cells stably expressing the indicated FLAG-Med26 proteins analyzed by silver staining (Ag stain) or western blotting (WB). (C) Med26(R61A,K62A)- Mediator fails to recruit EAF1 to a promoter. Assays contained template, HNF4α, EAF1, and wild type or Med26(R61A,K62A)-Mediator. (D) TFIID blocks Mediator-dependent recruitment of EAF1 to a promoter. Assays contained template, HNF4α, EAF1, TFIID and Mediator.
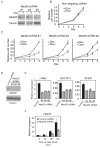
Figure 5. Effect of Med26 depletion on cell proliferation and gene expression
(A) Dox-inducible shRNA-mediated silencing of endogenous Med26 expression in 293T cells stably expressing different shRNAs. Cells were incubated with or without Dox for 48 hours, and cell lysates were analyzed by western blotting. (B)(C) Med26 depletion decreases cell proliferation in 293T cells stably expressing Dox-inducible shRNAs. Cell number is expressed relative to cell number at day 5 in cultures without Dox. (D) Western blots for endogenous Med26 or tubulin 48 hrs after transfection of non-targeting (control) or Med26 siRNAs. (E) Effect of siRNA-mediated Med26 depletion on gene expression. (F) Effect of Med26 depletion on Hsp70 induction by heat shock. Data points are the average of three independent experiments; error bars show standard deviation. See also Figure S2 and Table S2.
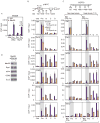
Figure 6. Effect of Med26 depletion on recruitment of SEC Components and Pol II CTD phosphorylation
(A) Occupancy of Med26 on the promoter of c-MYC or HSP70 gene under non-heat or heat shocked condition (7.5 min). (B)(C) Effect of Med26 depletion on AFF4, CDK9 and Pol II occupancy on the c-MYC or HSP70 gene under steady state or heat shocked conditions in HEK293T cells. Ig, IgG (TBP, Pol II, AFF4, CDK9 and Ser5p ChIPs) or IgM (Ser2p ChIP) control; Spec ab, specific antibody. (A)(B)(C) ChIP/input is average from two biological replicates, error bars show data range. (D) Med26, Pol II and SEC components in HEK293T cells treated with non-targeting siRNA or Med26 siRNA #4.
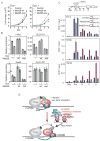
Figure 7. The Med26 NTD regulates cell proliferation and gene expression
(A) Med26-wt (wild type), but not Med26-mut (R61A,K62A), rescues proliferation defect of Med26 depleted 293T cells expressing Dox-inducible Med26 shRNA #3 and exogenous wild type or mutant Med26, grown with or without Dox. (B) Wild type, but not Med26(R61A,K62A) rescues gene expression defect of Med26 depleted cells. (C) Effect of overexpressing Med26 or Med26(R61A,K62A) on Pol II occupancy at c-MYC in HeLa cells stably expressing wild type or mutant Med26. Ig, IgG (Pol II and Ser5p ChIPs) or IgM (Ser2p ChIP) control; Spec ab, specific antibody. (D) Model for Mediator and Med26 NTD function during the transition to productive elongation. In the initiation and/or early elongation complexes, the EAF binding site on the Med26 NTD is occluded by interaction with TFIID. As Pol II moves further from the initiation site and/or upon receipt of a suitable signal, the Med26 NTD is released from TFIID, allowing it to recruit ELL/EAF complexes. Green and red oval, Med26; N, Med26 NTD; black bars, binding sites for Med26 NTD on TFIID and EAF family members.
Similar articles
- Multiple P-TEFbs cooperatively regulate the release of promoter-proximally paused RNA polymerase II.
Lu X, Zhu X, Li Y, Liu M, Yu B, Wang Y, Rao M, Yang H, Zhou K, Wang Y, Chen Y, Chen M, Zhuang S, Chen LF, Liu R, Chen R. Lu X, et al. Nucleic Acids Res. 2016 Aug 19;44(14):6853-67. doi: 10.1093/nar/gkw571. Epub 2016 Jun 28. Nucleic Acids Res. 2016. PMID: 27353326 Free PMC article. - MED26 regulates the transcription of snRNA genes through the recruitment of little elongation complex.
Takahashi H, Takigawa I, Watanabe M, Anwar D, Shibata M, Tomomori-Sato C, Sato S, Ranjan A, Seidel CW, Tsukiyama T, Mizushima W, Hayashi M, Ohkawa Y, Conaway JW, Conaway RC, Hatakeyama S. Takahashi H, et al. Nat Commun. 2015 Jan 9;6:5941. doi: 10.1038/ncomms6941. Nat Commun. 2015. PMID: 25575120 Free PMC article. - [Role for the human mediator subunit Med26 in transcription elongation].
Takahashi H. Takahashi H. Seikagaku. 2012 Jul;84(7):577-81. Seikagaku. 2012. PMID: 22991831 Review. Japanese. No abstract available. - Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila.
Ivaldi MS, Karam CS, Corces VG. Ivaldi MS, et al. Genes Dev. 2007 Nov 1;21(21):2818-31. doi: 10.1101/gad.1604007. Epub 2007 Oct 17. Genes Dev. 2007. PMID: 17942706 Free PMC article.
Cited by
- Mediator and SAGA have distinct roles in Pol II preinitiation complex assembly and function.
Chen XF, Lehmann L, Lin JJ, Vashisht A, Schmidt R, Ferrari R, Huang C, McKee R, Mosley A, Plath K, Kurdistani SK, Wohlschlegel J, Carey M. Chen XF, et al. Cell Rep. 2012 Nov 29;2(5):1061-7. doi: 10.1016/j.celrep.2012.10.019. Epub 2012 Nov 21. Cell Rep. 2012. PMID: 23177621 Free PMC article. - Multiple P-TEFbs cooperatively regulate the release of promoter-proximally paused RNA polymerase II.
Lu X, Zhu X, Li Y, Liu M, Yu B, Wang Y, Rao M, Yang H, Zhou K, Wang Y, Chen Y, Chen M, Zhuang S, Chen LF, Liu R, Chen R. Lu X, et al. Nucleic Acids Res. 2016 Aug 19;44(14):6853-67. doi: 10.1093/nar/gkw571. Epub 2016 Jun 28. Nucleic Acids Res. 2016. PMID: 27353326 Free PMC article. - Tail and Kinase Modules Differently Regulate Core Mediator Recruitment and Function In Vivo.
Jeronimo C, Langelier MF, Bataille AR, Pascal JM, Pugh BF, Robert F. Jeronimo C, et al. Mol Cell. 2016 Nov 3;64(3):455-466. doi: 10.1016/j.molcel.2016.09.002. Epub 2016 Oct 20. Mol Cell. 2016. PMID: 27773677 Free PMC article. - Mechanisms of Mediator complex action in transcriptional activation.
Ansari SA, Morse RH. Ansari SA, et al. Cell Mol Life Sci. 2013 Aug;70(15):2743-56. doi: 10.1007/s00018-013-1265-9. Epub 2013 Jan 30. Cell Mol Life Sci. 2013. PMID: 23361037 Free PMC article. Review. - Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells.
Giraud M, Yoshida H, Abramson J, Rahl PB, Young RA, Mathis D, Benoist C. Giraud M, et al. Proc Natl Acad Sci U S A. 2012 Jan 10;109(2):535-40. doi: 10.1073/pnas.1119351109. Epub 2011 Dec 27. Proc Natl Acad Sci U S A. 2012. PMID: 22203960 Free PMC article.
References
- Adelman K, Marr MT, Werner J, Saunders A, Ni Z, Andrulis ED, Lis JT. Efficient release from promoter-proximal stall sites requires transcript cleavage factor TFIIS. Mol Cell. 2005;17:103–112. - PubMed
- Andrau JC, van de PL, Lijnzaad P, Bijma T, Koerkamp MG, van de PJ, Werner M, Holstege FC. Genome-wide location of the coactivator mediator: Binding without activation and transient Cdk8 interaction on DNA. Mol Cell. 2006;22:179–192. - PubMed
- Banks CA, Kong SE, Spahr H, Florens L, Martin-Brown S, Washburn MP, Conaway JW, Mushegian A, Conaway RC. Identification and Characterization of a Schizosaccharomyces pombe RNA Polymerase II Elongation Factor with Similarity to the Metazoan Transcription Factor ELL. J Biol Chem. 2007;282:5761–5769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials