Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue - PubMed (original) (raw)
Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue
Jun Kurokawa et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Infiltration of inflammatory macrophages into adipose tissues with the progression of obesity triggers insulin resistance and obesity-related metabolic diseases. We recently reported that macrophage-derived apoptosis inhibitor of macrophage (AIM) protein is increased in blood in line with obesity progression and is incorporated into adipocytes, thereby inducing lipolysis in adipose tissue. Here we show that such a response is required for the recruitment of adipose tissue macrophages. In vitro, AIM-dependent lipolysis induced an efflux of palmitic and stearic acids from 3T3-L1 adipocytes, thereby stimulating chemokine production in adipocytes via activation of toll-like receptor 4 (TLR4). In vivo administration of recombinant AIM to TLR4-deficient (TLR4(-/-)) mice resulted in induction of lipolysis without chemokine production in adipose tissues. Consistently, mRNA levels for the chemokines that affect macrophages were far lower in AIM-deficient (AIM(-/-)) than in wild-type (AIM(+/+)) obese adipose tissue. This reduction in chemokine production resulted in a marked prevention of inflammatory macrophage infiltration into adipose tissue in obese AIM(-/-) mice, although these mice showed more advanced obesity than AIM(+/+) mice on a high-fat diet. Diminished macrophage infiltration resulted in decreased inflammation locally and systemically in obese AIM(-/-) mice, thereby protecting them from insulin resistance and glucose intolerance. These results indicate that the increase in blood AIM is a critical event for the initiation of macrophage recruitment into adipose tissue, which is followed by insulin resistance. Thus, AIM suppression might be therapeutically applicable for the prevention of obesity-related metabolic disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
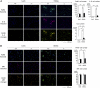
Requirement of AIM for macrophage recruitment into obese adipose tissue. (A and B) Specimens of epididymal fat tissue from lean (0 wk) or obese (fed a HFD for 12 wk) AIM+/+ and AIM−/− mice were costained for F4/80 (pan-macrophage marker; green), IL-6 (red), and Hoechst (blue) for A, and F4/80 (pan-macrophage marker; green), mannose receptor (MR) (red), and Hoechst (blue) for B. (Scale bar, 200 μm.) Quantification of F4/80+ cell number, IL-6+ macrophages, and the number of crown-like structures (CLS) are presented for A, or F4/80+ cell number and MR+ macrophages for B are presented. At least three different areas in three different sections per mouse were analyzed in six to eight mice of each genotype. Results are presented as averages ± SEM.
Fig. 2.
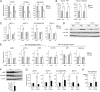
AIM-dependent lipolysis induces chemokine production in adipocytes via TLR4 stimulation. (A) Chemotaxis of RAW 264.1 cells in response to specified stimulant. Attractants: rAIM (25 μg/mL), C75 (25 μM), AIM CM/C75 CM: conditioned medium from 3T3-L1 adipocytes treated for 3 d with rAIM (25 μg/mL) or C75 (25 μM), respectively; AIM+αCD36 CM/AIM+IgA CM: conditioned medium from 3T3-L1 adipocytes treated for 3 d with rAIM (25 μg/mL) in the presence of anti-CD36 Ab or mouse IgA (10 μg/mL each), respectively; none CM, control CM: treated without rAIM or C75; and FM: fresh DMEM culture medium containing 10% FBS. Averages from n = 3 ± SEM. MCP-1 (100 ng/mL) was used as a positive control. (B) Degradation of IkBα in 3T3-L1 adipoctytes in response to specified stimulant in the absence (−) or presence (+) of a TIRAP inhibitor (100 μM). LPS (100 ng/mL) was used as a positive control. Representative immunoblotting results are presented. The density of the signal was quantified using National Institutes of Health Image J image analysis software and presented as values relative to those of prestimulation (Lower two panels). n = 3. Error bar: SEM. *, versus the value at prestimulation (0 min). (C) QPCR analysis of mRNA levels for MCP-1, CCL5/RANTES, MCP-2, and MCP-3 using RNA isolated from 3T3-L1 adipocytes treated with specified stimulant for 24 h in the absence (white bars) or presence (black bars) of a TIRAP inhibitor. Values were presented as relative expression to those without stimulation (none). n = 3 for each. Error bar: SEM. (D and E) No degradation of IkBα or expression induction of mRNA for chemokine genes in 3T3-L1 adipoctytes in response to rAIM alone (25 μg/mL) (D) or AIM+αCD36 CM (E).
Fig. 3.
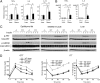
Involvement of TLR4 in adipose tissue macrophage recruitment by AIM in vivo. (A_–_E) TLR4−/− and wild-type littermate mice (B6 background) were i.v. injected with rAIM or BSA three times every other day (400 μg in 200 μL PBS per injection). The day after the third injection (day 8 from the first injection), mice were killed, and lipolysis, chemokine expression, and adipose tissue macrophage accumulation were analyzed. n = 5 for each. (A) mRNA levels for FSP27, Perilipin, and Adipophilin were assessed by QPCR using RNA isolated from epididymal fat. Values were presented as relative expression to those of fat tissue injected with BSA. Error bar: SEM. (B) Serum levels for FFA and glycerol. (C) mRNA levels for chemokines. (D) Immunoblotting for total and phosphorylated JNK in epididymal fat. Immunoblot for β-actin is also presented. Results from three mice for each group are presented. Note that comparable results were obtained in five independent mice in each group. (E) mRNA levels for F4/80 pan-macrophage marker, M1 and M2 macrophage markers to assess macrophage recruitment. (F) Immunoblotting for total and phosphorylated JNK using lysates obtained from epididymal fats of AIM+/+ and AIM−/− mice fed a HFD for 12 wk (n = 4–6). Relative values of phosphorylated JNK signals to total JNK are also presented (Lower graph). (G) QPCR analysis of mRNA levels for chemokine genes in epididymal fat tissue and (H) serum MCP-1 concentration in AIM+/+ and AIM−/− mice fed a HFD for 0 (lean) or 12 wk (obese); n = 6–8.
Fig. 4.
Prevented inflammation and normal insulin sensitivity in obese AIM−/− mice. (A) Local inflammation. QPCR analysis of mRNA levels for inflammatory cytokine genes in epididymal fat tissue from AIM+/+ or AIM−/− mice fed a HFD for 0 (lean) or 12 wk (obese). n = 6–8 for each group. Values were presented as relative expression to that in lean AIM+/+ mice. Error bar: SEM. (B) Systemic inflammation. Serum TNFα and IL-6 levels are the same as in A. (C) AIM−/− and AIM+/+ mice fed a HFD for 12 wk (three mice for each) were fasted for 5 h and treated with insulin (10 U/kg body weight) via i.p. injection. Within 15 min, epididymal fat, skeletal muscle (gastrocnemius), and liver were isolated and examined by immunoblotting for phosphorylated AKT (p-AKT), total AKT, phosphorylated GSK3β (p-GSK3β), total GSK3 (α and β), and β-actin. (D) Glucose tolerance test (GTT) and insulin tolerance test (ITT) performed on AIM+/+ and AIM−/− mice fed a HFD for 0 (lean) or 12 wk (obese); n = 6–8 for each group. For ITT, two panels including absolute blood glucose levels (Left) and % of the initial (time 0) glucose level (Right) are presented.
Similar articles
- Increased expression of macrophage-inducible C-type lectin in adipose tissue of obese mice and humans.
Ichioka M, Suganami T, Tsuda N, Shirakawa I, Hirata Y, Satoh-Asahara N, Shimoda Y, Tanaka M, Kim-Saijo M, Miyamoto Y, Kamei Y, Sata M, Ogawa Y. Ichioka M, et al. Diabetes. 2011 Mar;60(3):819-26. doi: 10.2337/db10-0864. Epub 2011 Jan 31. Diabetes. 2011. PMID: 21282371 Free PMC article. - Naringenin suppresses neutrophil infiltration into adipose tissue in high-fat diet-induced obese mice.
Tsuhako R, Yoshida H, Sugita C, Kurokawa M. Tsuhako R, et al. J Nat Med. 2020 Jan;74(1):229-237. doi: 10.1007/s11418-019-01332-5. Epub 2019 Jun 19. J Nat Med. 2020. PMID: 31218550 - Inhibiting Glycogen Synthase Kinase 3 Reverses Obesity-Induced White Adipose Tissue Inflammation by Regulating Apoptosis Inhibitor of Macrophage/CD5L-Mediated Macrophage Migration.
Wang L, Wang Y, Zhang C, Li J, Meng Y, Dou M, Noguchi CT, Di L. Wang L, et al. Arterioscler Thromb Vasc Biol. 2018 Sep;38(9):2103-2116. doi: 10.1161/ATVBAHA.118.311363. Arterioscler Thromb Vasc Biol. 2018. PMID: 30026270 - Macrophage recruitment in obese adipose tissue.
Bai Y, Sun Q. Bai Y, et al. Obes Rev. 2015 Feb;16(2):127-36. doi: 10.1111/obr.12242. Epub 2015 Jan 13. Obes Rev. 2015. PMID: 25586506 Free PMC article. Review. - Molecular mechanism of obesity-induced adipose tissue inflammation; the role of Mincle in adipose tissue fibrosis and ectopic lipid accumulation.
Tanaka M. Tanaka M. Endocr J. 2020 Feb 28;67(2):107-111. doi: 10.1507/endocrj.EJ19-0417. Epub 2019 Dec 19. Endocr J. 2020. PMID: 31852849 Review.
Cited by
- Chemokine gene polymorphisms association with increased risk of type 2 diabetes mellitus in Tatar ethnic group, Russia.
Kochetova OV, Avzaletdinova DS, Morugova TV, Mustafina OE. Kochetova OV, et al. Mol Biol Rep. 2019 Feb;46(1):887-896. doi: 10.1007/s11033-018-4544-6. Epub 2018 Dec 10. Mol Biol Rep. 2019. PMID: 30536157 - Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and type 2 diabetes.
Lee J. Lee J. Arch Pharm Res. 2013 Feb;36(2):208-22. doi: 10.1007/s12272-013-0023-8. Epub 2013 Feb 10. Arch Pharm Res. 2013. PMID: 23397293 Free PMC article. Review. - Inflammation and oxidative stress in obesity-related glomerulopathy.
Tang J, Yan H, Zhuang S. Tang J, et al. Int J Nephrol. 2012;2012:608397. doi: 10.1155/2012/608397. Epub 2012 Apr 5. Int J Nephrol. 2012. PMID: 22567283 Free PMC article. - Stabilization and augmentation of circulating AIM in mice by synthesized IgM-Fc.
Kai T, Yamazaki T, Arai S, Miyazaki T. Kai T, et al. PLoS One. 2014 May 7;9(5):e97037. doi: 10.1371/journal.pone.0097037. eCollection 2014. PLoS One. 2014. PMID: 24804991 Free PMC article. - Circulating CD5L is associated with cardiovascular events and all-cause mortality in individuals with chronic kidney disease.
Castelblanco E, Sarrias MR, Betriu À, Soldevila B, Barranco-Altirriba M, Franch-Nadal J, Valdivielso JM, Bermudez-Lopez M, Groop PH, Fernández E, Alonso N, Mauricio D. Castelblanco E, et al. Aging (Albany NY). 2021 Oct 10;13(19):22690-22709. doi: 10.18632/aging.203615. Epub 2021 Oct 10. Aging (Albany NY). 2021. PMID: 34629330 Free PMC article.
References
- Hotamisligil GS, Shargill N-S, Spiegelman B-M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. - PubMed
- Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases