Protein self-diffusion in crowded solutions - PubMed (original) (raw)
Protein self-diffusion in crowded solutions
Felix Roosen-Runge et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Macromolecular crowding in biological media is an essential factor for cellular function. The interplay of intermolecular interactions at multiple time and length scales governs a fine-tuned system of reaction and transport processes, including particularly protein diffusion as a limiting or driving factor. Using quasielastic neutron backscattering, we probe the protein self-diffusion in crowded aqueous solutions of bovine serum albumin on nanosecond time and nanometer length scales employing the same protein as crowding agent. The measured diffusion coefficient D(ϕ) strongly decreases with increasing protein volume fraction ϕ explored within 7% ≤ ϕ ≤ 30%. With an ellipsoidal protein model and an analytical framework involving colloid diffusion theory, we separate the rotational D(r)(ϕ) and translational D(t)(ϕ) contributions to D(ϕ). The resulting D(t)(ϕ) is described by short-time self-diffusion of effective spheres. Protein self-diffusion at biological volume fractions is found to be slowed down to 20% of the dilute limit solely due to hydrodynamic interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
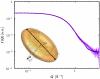
SAXS intensity for a dilute solution of BSA (5 mg/mL, room temperature) in 150 mM Hepes buffer after subtraction of background contributions. The data (circle) can be fitted with the form factor of an oblate ellipsoid (solid line). The deviation at higher Q is caused from the deviation of the protein shape from an ellipsoid at smaller length scales. The fitting of scattering data from several solutions with protein concentration below 10 mg/mL and varying concentration of Hepes buffer and NaCl is consistent with an oblate ellipsoid with polar semiaxis a ≈ 1.8 ± 0.05 nm and equatorial semiaxes b ≈ 4.6 ± 0.15 nm. This protein model of an oblate ellipsoid (inset lower left corner) is used as input for the further data analysis based on colloid theory.
Fig. 2.
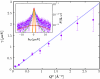
Inset: Example backscattering spectrum S(Q,ω) (symbols) recorded at IN16 for BSA in D2O (c = 500 mg/mL, φ = 28.5%, T = 300 K, individual detector at Q = 0.81 _Å_-1). The magenta solid line is the fit of the model from Eq. 3. The two Lorentzians in Eq. 3 are indicated by the dashed and dash-dotted lines. The orange solid line denotes the resolution function. Main figure: Fitted γ (symbols) vs. _Q_2 for the full _Q_-range of the example data. The fit of γ = D _Q_2 (blue line) is consistent with simple diffusive behavior. For statistical reasons the fit range is restricted to _Q_2 < 1.5 _Å_-2.
Fig. 3.
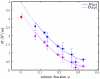
Measured diffusion coefficients D for BSA solutions in D2O at T = 280 K (blue circles on upper curve) and translational diffusion coefficients D t (purple circles on lower curve) computed from D using Eq. 4 and the theoretical rotational diffusion coefficient from ref. . The lines are polynomial fits. The dilute limit D t(0) (diamond symbol) is calculated from results of dynamic light scattering (41). The noncoincidence of D t(0) and the fit to D indicates a significant rotational contribution. After separation of the rotational contribution, the translational diffusion coefficient D t is in accordance with the dilute limit, supporting the validity of our approach.
Fig. 4.
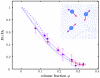
Normalized translational self-diffusion coefficients (Fig. 3) D t/D t(0) (circles) for two different temperatures (red and purple circles denote 280 and 300 K, respectively) after separation of the rotational contributions. The purple line superimposed on the data is a guide to the eye obtained from a polynomial fit indicating the temperature-independent master-curve. The upper and lower dashed purple lines indicate the upper and lower 96% prediction bounds, respectively. The blue lines denotes the colloidal short-time self-diffusion for hard spheres (light blue, solid) and charged spheres (dark blue, dashed). The inset in the upper right corner illustrates the flow field (light blue stream line plot) generated by the movement of three spheres (velocities are denoted by blue arrows) and therefore experiencing a hydrodynamic forces (pink arrows).
Similar articles
- Protein diffusion in crowded electrolyte solutions.
Roosen-Runge F, Hennig M, Seydel T, Zhang F, Skoda MW, Zorn S, Jacobs RM, Maccarini M, Fouquet P, Schreiber F. Roosen-Runge F, et al. Biochim Biophys Acta. 2010 Jan;1804(1):68-75. doi: 10.1016/j.bbapap.2009.07.003. Epub 2009 Jul 17. Biochim Biophys Acta. 2010. PMID: 19616646 - Short-Time Transport Properties of Bidisperse Suspensions of Immunoglobulins and Serum Albumins Consistent with a Colloid Physics Picture.
Beck C, Grimaldo M, Lopez H, Da Vela S, Sohmen B, Zhang F, Oettel M, Barrat JL, Roosen-Runge F, Schreiber F, Seydel T. Beck C, et al. J Phys Chem B. 2022 Sep 29;126(38):7400-7408. doi: 10.1021/acs.jpcb.2c02380. Epub 2022 Sep 16. J Phys Chem B. 2022. PMID: 36112146 Free PMC article. - Macromolecular crowding in biological systems: hydrodynamics and NMR methods.
Bernadó P, García de la Torre J, Pons M. Bernadó P, et al. J Mol Recognit. 2004 Sep-Oct;17(5):397-407. doi: 10.1002/jmr.694. J Mol Recognit. 2004. PMID: 15362098 Review. - Non-Gaussian, transiently anomalous, and ergodic self-diffusion of flexible dumbbells in crowded two-dimensional environments: Coupled translational and rotational motions.
Klett K, Cherstvy AG, Shin J, Sokolov IM, Metzler R. Klett K, et al. Phys Rev E. 2021 Dec;104(6-1):064603. doi: 10.1103/PhysRevE.104.064603. Phys Rev E. 2021. PMID: 35030844 - Formation of protein complexes in crowded environments--from in vitro to in vivo.
Phillip Y, Schreiber G. Phillip Y, et al. FEBS Lett. 2013 Apr 17;587(8):1046-52. doi: 10.1016/j.febslet.2013.01.007. Epub 2013 Jan 18. FEBS Lett. 2013. PMID: 23337873 Free PMC article. Review.
Cited by
- Beyond the excluded volume effects: mechanistic complexity of the crowded milieu.
Kuznetsova IM, Zaslavsky BY, Breydo L, Turoverov KK, Uversky VN. Kuznetsova IM, et al. Molecules. 2015 Jan 14;20(1):1377-409. doi: 10.3390/molecules20011377. Molecules. 2015. PMID: 25594347 Free PMC article. Review. - Dynamic cluster formation determines viscosity and diffusion in dense protein solutions.
von Bülow S, Siggel M, Linke M, Hummer G. von Bülow S, et al. Proc Natl Acad Sci U S A. 2019 May 14;116(20):9843-9852. doi: 10.1073/pnas.1817564116. Epub 2019 Apr 29. Proc Natl Acad Sci U S A. 2019. PMID: 31036655 Free PMC article. - Conformational dynamics of a multidomain protein by neutron scattering and computational analysis.
Nakagawa H, Saio T, Nagao M, Inoue R, Sugiyama M, Ajito S, Tominaga T, Kawakita Y. Nakagawa H, et al. Biophys J. 2021 Aug 17;120(16):3341-3354. doi: 10.1016/j.bpj.2021.07.001. Epub 2021 Jul 7. Biophys J. 2021. PMID: 34242590 Free PMC article. - Slow-Down in Diffusion in Crowded Protein Solutions Correlates with Transient Cluster Formation.
Nawrocki G, Wang PH, Yu I, Sugita Y, Feig M. Nawrocki G, et al. J Phys Chem B. 2017 Dec 14;121(49):11072-11084. doi: 10.1021/acs.jpcb.7b08785. Epub 2017 Nov 30. J Phys Chem B. 2017. PMID: 29151345 Free PMC article. - Stacking Interactions and Flexibility of Human Telomeric Multimers.
Rosi BP, Libera V, Bertini L, Orecchini A, Corezzi S, Schirò G, Pernot P, Biehl R, Petrillo C, Comez L, De Michele C, Paciaroni A. Rosi BP, et al. J Am Chem Soc. 2023 Jul 26;145(29):16166-16175. doi: 10.1021/jacs.3c04810. Epub 2023 Jul 11. J Am Chem Soc. 2023. PMID: 37432645 Free PMC article.
References
- Ellis RJ. Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr Opin Struct Biol. 2001;11:114–119. - PubMed
- Zimmerman SB, Minton AP. Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annu Rev Biophys Biomol Struct. 1993;22:27–65. - PubMed
- Purcell EM. Life at low Reynolds number. Am J Phys. 1977;45:3–11.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources