Microglial p38α MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ) - PubMed (original) (raw)
Microglial p38α MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ)
Adam D Bachstetter et al. J Neuroinflammation. 2011.
Abstract
Background: Overproduction of proinflammatory cytokines from activated microglia has been implicated as an important contributor to pathophysiology progression in both acute and chronic neurodegenerative diseases. Therefore, it is critical to elucidate intracellular signaling pathways that are significant contributors to cytokine overproduction in microglia exposed to specific stressors, especially pathways amenable to drug interventions. The serine/threonine protein kinase p38α MAPK is a key enzyme in the parallel and convergent intracellular signaling pathways involved in stressor-induced production of IL-1β and TNFα in peripheral tissues, and is a drug development target for peripheral inflammatory diseases. However, much less is known about the quantitative importance of microglial p38α MAPK in stressor-induced cytokine overproduction, or the potential of microglial p38α MAPK to be a druggable target for CNS disorders. Therefore, we examined the contribution of microglial p38αMAPK to cytokine up-regulation, with a focus on the potential to suppress the cytokine increase by inhibition of the kinase with pharmacological or genetic approaches.
Methods: The microglial cytokine response to TLR ligands 2/3/4/7/8/9 or to Aβ1-42 was tested in the presence of a CNS-penetrant p38α MAPK inhibitor, MW01-2-069A-SRM. Primary microglia from mice genetically deficient in p38α MAPK were used to further establish a linkage between microglia p38α MAPK and cytokine overproduction. The in vivo significance was determined by p38α MAPK inhibitor treatment in a LPS-induced model of acute neuroinflammation.
Results: Increased IL-1β and TNFα production by the BV-2 microglial cell line and by primary microglia cultures was inhibited in a concentration-dependent manner by the p38α MAPK-targeted inhibitor. Cellular target engagement was demonstrated by the accompanying decrease in the phosphorylation state of two p38α MAPK protein substrates, MK2 and MSK1. Consistent with the pharmacological findings, microglia from p38α-deficient mice showed a diminished cytokine response to LPS. Further, oral administration of the inhibitor blocked the increase of IL-1β in the cerebral cortex of mice stressed by intraperitoneal injection of LPS.
Conclusion: The p38α MAPK pathway is an important contributor to the increased microglial production of proinflammatory cytokines induced by diverse stressors. The results also indicate the feasibility of targeting p38α MAPK to modulate CNS proinflammatory cytokine overproduction.
Figures
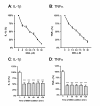
Figure 1
The small molecule p38α MAPK inhibitor, 069A, suppresses the LPS-induced increase in cytokine production by the BV-2 microglia cell line. BV-2 cells were stimulated with 100ng/ml of LPS in the absence or presence of increasing concentrations of 069A (0.9 - 30 μM). 069A inhibited LPS-induced IL-1β (A) and TNFα (B) production in a concentration-dependent manner, with an IC50 of 3.7 μM and 4.5 μM, respectively. Compound 069A (4 μM) was equally as effective in blocking the IL-1β (C) and TNFα (D) cytokine response to LPS when the compound was given at the same time as LPS (time 0) or when given at different times after LPS exposure. White bars show the response of the LPS stimulated cells in the absence of 069A. Gray bars show the response to LPS in the presence of 069A. Data are expressed as a percent of the maximal activity, where activity in the presence of LPS alone is taken as 100%. Asterisk denotes significance (*** = p < 0.001) in response to LPS in the absence (white bars) or presence (gray bars) of 069A.
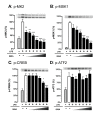
Figure 2
The p38α inhibitor, 069A, selectively inhibits phosphorylation of the p38α substrates MK2 and MSK1 in LPS-stimulated BV-2 microglia cells. BV-2 cells were stimulated with 100ng/ml of LPS in the absence or presence of increasing concentrations of 069A (0.9 - 30 μM). Levels of (A) p-MK2 and (B) p-MSK1 were suppressed by the p38α inhibitor in a concentration-dependent manner. Treatment of cells with 069A had an effect on p-CREB (C) only at the two highest compound concentrations, and had no effect on p-ATF2 levels (D). Gray bars show the response of control, unstimulated cells; white bars show the response of LPS-stimulated cells in the absence of 069A; black bars show the response of LPS-stimulated cells in the presence of 069A. For each endpoint, data are expressed as a percent of the maximal activity, where activity in the presence of LPS alone is taken as 100%. Asterisk denotes significance (* = p < 0.05, ** = p < 0.01, or *** = p < 0.001) in endpoint for LPS-stimulated cells (white bars) to LPS-stimulated cells in presence of 069A (black bars). Data represent five independent experiments.
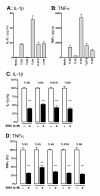
Figure 3
The p38α MAPK inhibitor 069A attenuates the IL-1β and TNFα increase in response to diverse TLR ligands. The IL-1β (A) and TNFα (B) response from BV-2 cells was measured after 18 hrs of stimulation with either: (TLR2) 10 μg/ml LTA; (TLR3) 50 μg/ml poly(I:C); (TLR4) 100ng/ml LPS; (TLR7/8) 500ng/ml CL097; or (TLR9) 500ng/ml ODN1668. Treatment of cells with 4 μM of the p38α inhibitor, 069A, led to a significant reduction in the levels of IL-1β (C) and TNFα (D) in response to each of the different TLR ligands. The white bar represents the BV-2 cells stimulated with the ligand but without 069A (normalized to 100%). The black bar represents the BV-2 cells treated with TLR ligand + 069A. Asterisk denotes significance (* = p < 0.05, ** = p < 0.01, or *** = p < 0.001) for ligand-stimulated without 069A (white bar) compared to stimulated with 069A (black bar). Data represent two independent experiments.
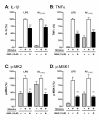
Figure 4
The p38α MAPK pathway is engaged in primary microglia and contributes to cytokine upregulation. Rat primary microglia were treated with either diluent (gray bars), stressor (either 1ng/ml LPS or 5 μM oligomeric Aβ1-42) in the absence of 069A (white bars), or stressor in the presence of 15 μM 069A (black bars). The LPS-induced increases in IL-1β (A) and TNFα (B) levels by rat primary microglia were significantly inhibited by the p38α inhibitor 069A. Similar results were obtained with a non-TLR ligand, Aβ1-42. LPS or Aβ also caused an increase in the phosphorylated (active) form of two p38α substrates, p-MK2 (C) and p-MSK1 (D), and phosphorylation of these substrates was blocked by the addition of 069A. Asterisk denotes significance (* = p < 0.05, ** = p < 0.01, or *** = p < 0.001) for stressor-stimulated microglia in the absence of 069A compared to stimulated microglia in the presence of 069A. Values with stimulus alone (white bars) were normalized to 100%. Data represent four independent experiments.
Figure 5
Microglia deficient in p38α MAPK show a reduced cytokine response to LPS. Primary microglia isolated from either p38α conditional KO mice (p38α-/-; black bar) or wild-type littermates (p38α+/+; white bar) were stimulated with 1ng/ml LPS for either 18 hrs (for cytokine measurements) or 1 hr (for western blots). There was a significant reduction in the IL-1β (A) and TNFα (B) response to LPS in microglia from the p38α KO mice compared to wild-type microglia. (C) There was also substantially reduced LPS-induced phosphorylation of p38α substrates MSK1 and MK2 in p38α-/- microglia compared to p38α+/+ microglia. The loss of p38α in the KO microglia was confirmed by little or no reactivity with a phospho-p38α/β antibody, a p38α/β antibody and a p38α-selective antibody. Asterisk denotes significance (*** = p < 0.001) for p38α+/+ microglia (white bar) compared to p38α-/- microglia (black bar). Responses of the wild-type microglia were normalized to 100%. Data represent three independent experiments.
Figure 6
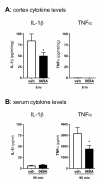
Oral administration of 069A suppresses LPS-induced cortical IL-1β and serum TNFα in vivo: C57Bl/6 mice were administered either saline vehicle or 069A (5mg/kg) by oral gavage one hr prior to an intraperitoneal LPS (1mg/kg) injection. Serum and cortex were harvested at 90 min and 6 hrs, respectively, after LPS injection. (A) At the 6 hr timepoint, IL-1β levels were significantly lower in the cortex of mice treated with 069A compared to vehicle-treated mice. TNFα levels in the cortex were at or near the limit of detection of the assay. (B) At the 90 min timepoint, TNFα levels were significantly lower in the serum of mice treated with 069A compared to vehicle-treated mice. Serum IL-1β levels were low, and there was no inhibition by 069A at this timepoint. Asterisk denotes significance (* = p < 0.05) for vehicle-treated mice (white bar) compared to 069A-treated mice (black bar). N = 5-8 per group.
Similar articles
- Microglial p38α MAPK is critical for LPS-induced neuron degeneration, through a mechanism involving TNFα.
Xing B, Bachstetter AD, Van Eldik LJ. Xing B, et al. Mol Neurodegener. 2011 Dec 20;6:84. doi: 10.1186/1750-1326-6-84. Mol Neurodegener. 2011. PMID: 22185458 Free PMC article. - A novel p38 alpha MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer's disease mouse model.
Munoz L, Ralay Ranaivo H, Roy SM, Hu W, Craft JM, McNamara LK, Chico LW, Van Eldik LJ, Watterson DM. Munoz L, et al. J Neuroinflammation. 2007 Sep 4;4:21. doi: 10.1186/1742-2094-4-21. J Neuroinflammation. 2007. PMID: 17784957 Free PMC article. - Deficiency in p38β MAPK fails to inhibit cytokine production or protect neurons against inflammatory insult in in vitro and in vivo mouse models.
Xing B, Bachstetter AD, Van Eldik LJ. Xing B, et al. PLoS One. 2013;8(2):e56852. doi: 10.1371/journal.pone.0056852. Epub 2013 Feb 15. PLoS One. 2013. PMID: 23457629 Free PMC article. - The p38alpha mitogen-activated protein kinase as a central nervous system drug discovery target.
Borders AS, de Almeida L, Van Eldik LJ, Watterson DM. Borders AS, et al. BMC Neurosci. 2008 Dec 3;9 Suppl 2(Suppl 2):S12. doi: 10.1186/1471-2202-9-S2-S12. BMC Neurosci. 2008. PMID: 19090985 Free PMC article. Review. - p38α Mitogen-Activated Protein Kinase-An Emerging Drug Target for the Treatment of Alzheimer's Disease.
Detka J, Płachtij N, Strzelec M, Manik A, Sałat K. Detka J, et al. Molecules. 2024 Sep 13;29(18):4354. doi: 10.3390/molecules29184354. Molecules. 2024. PMID: 39339348 Free PMC article. Review.
Cited by
- How Do Post-Translational Modifications Influence the Pathomechanistic Landscape of Huntington's Disease? A Comprehensive Review.
Lontay B, Kiss A, Virág L, Tar K. Lontay B, et al. Int J Mol Sci. 2020 Jun 16;21(12):4282. doi: 10.3390/ijms21124282. Int J Mol Sci. 2020. PMID: 32560122 Free PMC article. Review. - Mild cognitive impairment and major depressive disorder are associated with molecular senescence abnormalities in older adults.
Diniz BS, Vieira EM, Mendes-Silva AP, Bowie CR, Butters MA, Fischer CE, Flint A, Herrmann N, Kennedy J, Lanctôt KL, Mah L, Pollock BG, Mulsant BH, Rajji TK; on behalf of the PACt‐MD Study Group. Diniz BS, et al. Alzheimers Dement (N Y). 2021 Mar 31;7(1):e12129. doi: 10.1002/trc2.12129. eCollection 2021. Alzheimers Dement (N Y). 2021. PMID: 33816758 Free PMC article. - Nrf2 Signaling Pathway: Focus on Oxidative Stress in Spinal Cord Injury.
Xiao CL, Lai HT, Zhou JJ, Liu WY, Zhao M, Zhao K. Xiao CL, et al. Mol Neurobiol. 2024 Aug 2. doi: 10.1007/s12035-024-04394-z. Online ahead of print. Mol Neurobiol. 2024. PMID: 39093381 Review. - Exploratory Assessment of Proteomic Network Changes in Cerebrospinal Fluid of Mild Cognitive Impairment Patients: A Pilot Study.
Kamalian A, Ho SG, Patel M, Lewis A, Bakker A, Albert M, O'Brien RJ, Moghekar A, Lutz MW. Kamalian A, et al. Biomolecules. 2023 Jul 8;13(7):1094. doi: 10.3390/biom13071094. Biomolecules. 2023. PMID: 37509130 Free PMC article. - Age-associated alterations in the time-dependent profile of pro- and anti-inflammatory proteins within the hippocampus in response to acute exposure to interleukin-1β.
Hopp SC, Royer S, Brothers HM, Kaercher RM, D'Angelo H, Bardou I, Wenk GL. Hopp SC, et al. J Neuroimmunol. 2014 Feb 15;267(1-2):86-91. doi: 10.1016/j.jneuroim.2013.12.010. Epub 2013 Dec 22. J Neuroimmunol. 2014. PMID: 24393520 Free PMC article.
References
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG031311/AG/NIA NIH HHS/United States
- F32 AG037280/AG/NIA NIH HHS/United States
- R01 NS064247/NS/NINDS NIH HHS/United States
- R01 NS056051/NS/NINDS NIH HHS/United States
- F32 AG037280-01/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources