The trypanocidal drug suramin and other trypan blue mimetics are inhibitors of pyruvate kinases and bind to the adenosine site - PubMed (original) (raw)
The trypanocidal drug suramin and other trypan blue mimetics are inhibitors of pyruvate kinases and bind to the adenosine site
Hugh P Morgan et al. J Biol Chem. 2011.
Abstract
Ehrlich's pioneering chemotherapeutic experiments published in 1904 (Ehrlich, P., and Shiga, K. (1904) Berlin Klin. Wochenschrift 20, 329-362) described the efficacy of a series of dye molecules including trypan blue and trypan red to eliminate trypanosome infections in mice. The molecular structures of the dyes provided a starting point for the synthesis of suramin, which was developed and used as a trypanocidal drug in 1916 and is still in clinical use. Despite the biological importance of these dye-like molecules, the mode of action on trypanosomes has remained elusive. Here we present crystal structures of suramin and three related dyes in complex with pyruvate kinases from Leishmania mexicana or from Trypanosoma cruzi. The phenyl sulfonate groups of all four molecules (suramin, Ponceau S, acid blue 80, and benzothiazole-2,5-disulfonic acid) bind in the position of ADP/ATP at the active sites of the pyruvate kinases (PYKs). The binding positions in the two different trypanosomatid PYKs are nearly identical. We show that suramin competitively inhibits PYKs from humans (muscle, tumor, and liver isoenzymes, K(i) = 1.1-17 μM), T. cruzi (K(i) = 108 μM), and L. mexicana (K(i) = 116 μM), all of which have similar active sites. Synergistic effects were observed when examining suramin inhibition in the presence of an allosteric effector molecule, whereby IC(50) values decreased up to 2-fold for both trypanosomatid and human PYKs. These kinetic and structural analyses provide insight into the promiscuous inhibition observed for suramin and into the mode of action of the dye-like molecules used in Ehrlich's original experiments.
Figures
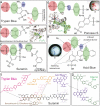
FIGURE 1.
Trypan dyes and other sulfonic acid compounds have similar structural features. a, two-dimensional representation of one symmetrical half (as indicated by the dashed symmetry line) of trypan blue and trypan red (trypan red contains one additional sulfono group, indicated by red lettering). b, BDS bound in the active site of _Lm_PYK (i). The BDS molecule is shown with an unbiased Fo − Fc electron density map contoured at 3 σ (green). Hydrogen bonds are shown as dashed red lines, and stacking interactions are shown as dashed purple lines. c, one symmetrical half of the colorless trypan analog, suramin. d, co-crystal (i) of Ponceau S bound in the active site of _Tc_PYK (ii). e, co-crystal (i) of _Lm_PYK and the AB80 molecule. f, a superimposition between dye-like molecules and suramin. Common groups superimpose onto the structure of suramin, highlighting the chemical relationships within the series of molecules. This figure highlights two-dimensional chemical similarities and not necessarily three-dimensional binding similarities (as illustrated by the shaded ellipses in panels a–e). g, a superimposition between Cibacron blue (a molecule commonly used for affinity purification of PYK) and AB80. Shaded ellipses in panels a–e highlight key binding characteristics observed in the _Lm_PYK-sulfonic acid structures obtained: site S1, pink ellipse, a sulfono group (S(i)) is observed bound in a near identical position in all complexes; site S2, green ellipse, a stacking interaction with His-54 is conserved in all structures; site S3, green ellipse, a second stacking interaction with Tyr-59 and Pro-29 is observed for suramin, Ponceau S, and AB80; site S4, blue ellipse, a hydrogen bound acceptor/donor is also commonly observed. In the _Lm_PYK-Ponceau S, the azo group forms a hydrogen bond via the hydroxyl group of Tyr-59. Only microcrystals were obtained with trypan blue, but the common PYK binding characteristics are also observed. Suramin and trypan blue are symmetrical molecules, and only half the molecule is shown (as indicated by the dashed symmetry lines).
FIGURE 2.
Structures of _Lm_PYK-suramin and _Lm_PYK-AB80 complexes. a, the _Lm_PYK-suramin tetramer in which the domains have been colored to aid identification (green, A domain; blue, B domain; red, C domain; yellow, N-domain). b, enlargement of the active site of the _Lm_PYK-suramin structure. Suramin is shown by cyan sticks and corresponds primarily to one symmetrical half of the suramin molecule. The position of the suramin molecule is shown by an unbiased Fo − Fc electron density map contoured at 2 σ (orange). A K+ ion is shown as a purple sphere. c, AB80 bound in the active site of _Lm_PYK. The AB80 molecule is shown with an unbiased Fo − Fc electron density map contoured at 2.5 σ (green). d, the _Lm_PYK-AB80 monomer (colored gray) superposed onto the _Lm_PYK-suramin structure (colored yellow); the orientation is identical to that of panel c. Overlapping binding characteristics of the sulfono group-containing molecules highlight key groups required for inhibitor binding; for clarity, only suramin and AB80 are shown. The four key binding characteristics observed in the _Lm_PYK and _Tc_PYK sulfonic acid structures are highlighted with colored ellipses as described previously in the legend for Fig. 1: site S1, pink ellipse, a sulfono group (S(i)) is observed bound in a near identical position in all complexes; site S2, green ellipse, a stacking interaction with His-54 is conserved in all structures; site S3, green ellipse, a second stacking interaction with Tyr-59 and Pro-29 is observed for suramin, Ponceau S, and AB80; site S4, blue ellipse, a hydrogen bound acceptor/donor is also commonly observed. Hydrogen bonds are shown as dashed red lines, and stacking interactions are shown as dashed purple lines.
FIGURE 3.
Suramin inhibition of PYK is enhanced in the presence of the activator molecule. a, a schematic drawing showing the interatomic distances (given in Å, dotted black lines) for the interactions shown in Fig. 2_b_). The solid purple lines indicate stacking interactions. Residues involved in ATP binding (orange box) and not involved (green) have been indicated. Only one symmetrical half of the suramin molecule is shown (as indicated by the dashed symmetry line). b, schematic representation of the inactive _Lm_PYK-suramin structure. Suramin is unambiguously (yellow lozenges) bound to one of the chains in the asymmetric unit (two chains outlined in black) but is disordered (blue square) in the adjacent chain, separated by the large (A-A) interface. A crystallographic two-fold axis running along the small interface generates two more chains (purple outlines), forming the biologically relevant tetramer structure. See Ref. for schematics of the enzyme in its other conformations. c and d, concentration-response curves observed for the titration of suramin against PYK activity in the absence (c) and presence (d) of activator (F16BP for human PYKs, F26BP for _Lm_PYK), using a luciferase-based assay. The values are expressed ± S.D. Suramin showed no inhibition in the control assay of luciferase activity alone. All assays were performed in triplicate. e, the binding of suramin prevents ADP/ATP binding in trypanosomatid PYKs. The R-state _Lm_PYK-ATP/oxalate/F26BP monomer (colored blue, PDB ID = 3HQP) was superposed onto the inactive _Lm_PYK-suramin structure (colored green). The A- and C-domains (residues 18–86 and 188–480) of both structures were superposed (r.m.s. fit of the C-α atoms of domains A and C is 0.50 Å). The superpositions of the ATP and suramin molecules clash, indicating a clear mechanism of competitive inhibition. f, the electrostatic surface of the suramin binding site, showing areas of positive charge (blue), which interact with the negatively charged sulfono groups of suramin. Two hydrophobic groups (Pro-29 and Tyr-59) provide further stability, helping to hold the molecule in the ATP/ADP binding site.
Similar articles
- The phosphoglycerate kinases from Trypanosoma brucei. A comparison of the glycosomal and the cytosolic isoenzymes and their sensitivity towards suramin.
Misset O, Opperdoes FR. Misset O, et al. Eur J Biochem. 1987 Feb 2;162(3):493-500. doi: 10.1111/j.1432-1033.1987.tb10667.x. Eur J Biochem. 1987. PMID: 3830152 - Inhibition of tumor necrosis factor-alpha (TNF-alpha)/TNF-alpha receptor binding by structural analogues of suramin.
Mancini F, Toro CM, Mabilia M, Giannangeli M, Pinza M, Milanese C. Mancini F, et al. Biochem Pharmacol. 1999 Sep 1;58(5):851-9. doi: 10.1016/s0006-2952(99)00150-1. Biochem Pharmacol. 1999. PMID: 10449196 - Squalene synthase as a chemotherapeutic target in Trypanosoma cruzi and Leishmania mexicana.
Urbina JA, Concepcion JL, Rangel S, Visbal G, Lira R. Urbina JA, et al. Mol Biochem Parasitol. 2002 Nov-Dec;125(1-2):35-45. doi: 10.1016/s0166-6851(02)00206-2. Mol Biochem Parasitol. 2002. PMID: 12467972 - Dyes, trypanosomiasis and DNA: a historical and critical review.
Wainwright M. Wainwright M. Biotech Histochem. 2010 Dec;85(6):341-54. doi: 10.3109/10520290903297528. Biotech Histochem. 2010. PMID: 21080764 Review. - The rational design of trypanocidal drugs: selective inhibition of the glyceraldehyde-3-phosphate dehydrogenase in Trypanosomatidae.
Callens M, Hannaert V. Callens M, et al. Ann Trop Med Parasitol. 1995 Dec;89 Suppl 1:23-30. doi: 10.1080/00034983.1995.11813011. Ann Trop Med Parasitol. 1995. PMID: 8745924 Review.
Cited by
- Crystal structure of Cryptosporidium parvum pyruvate kinase.
Cook WJ, Senkovich O, Aleem K, Chattopadhyay D. Cook WJ, et al. PLoS One. 2012;7(10):e46875. doi: 10.1371/journal.pone.0046875. Epub 2012 Oct 9. PLoS One. 2012. PMID: 23056503 Free PMC article. - Structures of pyruvate kinases display evolutionarily divergent allosteric strategies.
Morgan HP, Zhong W, McNae IW, Michels PA, Fothergill-Gilmore LA, Walkinshaw MD. Morgan HP, et al. R Soc Open Sci. 2014 Sep 24;1(1):140120. doi: 10.1098/rsos.140120. eCollection 2014 Sep. R Soc Open Sci. 2014. PMID: 26064527 Free PMC article. - Bactericidal bissulfone B7 targets bacterial pyruvate kinase to impair bacterial biology and pathogenicity in plants.
Zhang A, Zhang H, Wang R, He H, Song B, Song R. Zhang A, et al. Sci China Life Sci. 2024 Feb;67(2):391-402. doi: 10.1007/s11427-023-2449-1. Epub 2023 Nov 17. Sci China Life Sci. 2024. PMID: 37987940 - In silico approaches supporting drug repurposing for Leishmaniasis: a scoping review.
Scheiffer G, Domingues KZA, Gorski D, Cobre AF, Lazo REL, Borba HHL, Ferreira LM, Pontarolo R. Scheiffer G, et al. EXCLI J. 2024 Sep 3;23:1117-1169. doi: 10.17179/excli2024-7552. eCollection 2024. EXCLI J. 2024. PMID: 39421030 Free PMC article. Review. - Ethyl Pyruvate Emerges as a Safe and Fast Acting Agent against Trypanosoma brucei by Targeting Pyruvate Kinase Activity.
Worku N, Stich A, Daugschies A, Wenzel I, Kurz R, Thieme R, Kurz S, Birkenmeier G. Worku N, et al. PLoS One. 2015 Sep 4;10(9):e0137353. doi: 10.1371/journal.pone.0137353. eCollection 2015. PLoS One. 2015. PMID: 26340747 Free PMC article.
References
- Ehrlich P., Shiga K. (1904) Berlin Klin. Wochenschrift 20, 329–362
- Hawking F. (1978) Adv. Pharmacol. Chemother. 15, 289–322 - PubMed
- La Rocca R. V., Stein C. A., Danesi R., Myers C. E. (1990) J. Steroid Biochem. Mol. Biol. 37, 893–898 - PubMed
- Mahoney C. W., Azzi A., Huang K. P. (1990) J. Biol. Chem. 265, 5424–5428 - PubMed
- Mitsuya H., Popovic M., Yarchoan R., Matsushita S., Gallo R. C., Broder S. (1984) Science 226, 172–174 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources