Pathogenesis and pathophysiology of pneumococcal meningitis - PubMed (original) (raw)
Review
Pathogenesis and pathophysiology of pneumococcal meningitis
Barry B Mook-Kanamori et al. Clin Microbiol Rev. 2011 Jul.
Abstract
Pneumococcal meningitis continues to be associated with high rates of mortality and long-term neurological sequelae. The most common route of infection starts by nasopharyngeal colonization by Streptococcus pneumoniae, which must avoid mucosal entrapment and evade the host immune system after local activation. During invasive disease, pneumococcal epithelial adhesion is followed by bloodstream invasion and activation of the complement and coagulation systems. The release of inflammatory mediators facilitates pneumococcal crossing of the blood-brain barrier into the brain, where the bacteria multiply freely and trigger activation of circulating antigen-presenting cells and resident microglial cells. The resulting massive inflammation leads to further neutrophil recruitment and inflammation, resulting in the well-known features of bacterial meningitis, including cerebrospinal fluid pleocytosis, cochlear damage, cerebral edema, hydrocephalus, and cerebrovascular complications. Experimental animal models continue to further our understanding of the pathophysiology of pneumococcal meningitis and provide the platform for the development of new adjuvant treatments and antimicrobial therapy. This review discusses the most recent views on the pathophysiology of pneumococcal meningitis, as well as potential targets for (adjunctive) therapy.
Figures
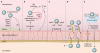
Fig. 1.
(A) Mucus breakdown. S. pneumoniae colonization of the nasopharynx is facilitated by mucus degradation by the enzymes NanA, BgaA, StrH, and NanB. Ply decreases epithelial cell ciliary beating, enhancing bacterial adherence. (B) Evasion of proteolytic enzymes. Pneumococcal cell wall peptidoglycans may be destroyed by lysozyme. PdgA and Adr deacetylate pneumococcal cell surface petidoglycan molecules, rendering them resistant to lysozyme. (C) Epithelial cell binding. S. pneumoniae binds host GalNac by using SpxB, Smi, MsrA, and PlpA. (D) Intracellular translocation. By binding the pIgR with PspC (or PAF receptor [PAFr] with ChoP), pneumococci can use the pIgR or PAF receptor recycling pathway to be transported through the epithelial cell layer. (E) Inter- and pericellular translocation. Plasminogen bound by Gly3Ph, CbpE, and enolase enhances epithelial cell binding and degrades interepithelial adherens junctions, allowing pericellular migration.
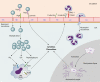
Fig. 2.
S. pneumoniae adheres to endothelial cells by using PspC, which binds laminin and pIgR, enabling transcytosis across the endothelium. Once in the CSF, pneumococci multiply freely and release bacterial products such as LTA and Ply, which are recognized by TLR2 and TLR4 on circulating APCs. The subsequent release of proinflammatory cytokines and chemokines from macrophages and microglial cells results in upregulation of endothelial cell P- and E-selectin and ICAM (which binds MAC-1 on leukocytes), leading to increased neutrophil recruitment into the CSF.
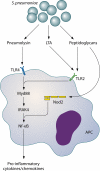
Fig. 3.
Host pattern recognition receptors involved in sensing S. pneumoniae. TLR2 is activated by pneumococcal cell wall peptidoglycan and LTA. Nod2 is activated by cell wall peptidoglycans and TLR4, which in turn is activated by Ply. TLR2 and -4 activate the transcription factor NF-κB via MyD88 and IRAK-4. Nod2 also activates NF-κB, inducing transcription of several proinflammatory cytokines.
Fig. 4.
Neuronal damage and histopathology in humans with pneumococcal meningitis. The images show the histopathology of patients with bacterial meningitis, including parenchymal and meningeal hemorrhages (A), neutrophilic infiltration and arteritis obliterans (B), abscess formation and venous thrombosis (C), recent infarctions (D and E), and meningitis without cortical infiltration (F).
Similar articles
- Blood‒Brain Barrier Pathology and CNS Outcomes in Streptococcus pneumoniae Meningitis.
Yau B, Hunt NH, Mitchell AJ, Too LK. Yau B, et al. Int J Mol Sci. 2018 Nov 11;19(11):3555. doi: 10.3390/ijms19113555. Int J Mol Sci. 2018. PMID: 30423890 Free PMC article. Review. - Pathogenesis and pathophysiology of pneumococcal meningitis.
Koedel U, Scheld WM, Pfister HW. Koedel U, et al. Lancet Infect Dis. 2002 Dec;2(12):721-36. doi: 10.1016/s1473-3099(02)00450-4. Lancet Infect Dis. 2002. PMID: 12467688 Review. - Pathophysiology of acute meningitis caused by Streptococcus pneumoniae and adjunctive therapy approaches.
Barichello T, Generoso JS, Collodel A, Moreira AP, Almeida SM. Barichello T, et al. Arq Neuropsiquiatr. 2012 May;70(5):366-72. doi: 10.1590/s0004-282x2012000500011. Arq Neuropsiquiatr. 2012. PMID: 22618789 Review. - Advances in the pathogenesis and treatment of pneumococcal meningitis.
Xu Y, Wang J, Qin X, Liu J. Xu Y, et al. Virulence. 2024 Dec;15(1):2387180. doi: 10.1080/21505594.2024.2387180. Epub 2024 Aug 27. Virulence. 2024. PMID: 39192572 Free PMC article. Review. - Impact of bacteremia on the pathogenesis of experimental pneumococcal meningitis.
Brandt CT, Holm D, Liptrot M, Ostergaard C, Lundgren JD, Frimodt-Møller N, Skovsted IC, Rowland IJ. Brandt CT, et al. J Infect Dis. 2008 Jan 15;197(2):235-44. doi: 10.1086/524874. J Infect Dis. 2008. PMID: 18173365
Cited by
- Host-microbe interactions at the blood-brain barrier through the lens of induced pluripotent stem cell-derived brain-like endothelial cells.
Vollmuth N, Sin J, Kim BJ. Vollmuth N, et al. mBio. 2024 Feb 14;15(2):e0286223. doi: 10.1128/mbio.02862-23. Epub 2024 Jan 9. mBio. 2024. PMID: 38193670 Free PMC article. Review. - Infection of zebrafish embryos with live fluorescent Streptococcus pneumoniae as a real-time pneumococcal meningitis model.
Jim KK, Engelen-Lee J, van der Sar AM, Bitter W, Brouwer MC, van der Ende A, Veening JW, van de Beek D, Vandenbroucke-Grauls CM. Jim KK, et al. J Neuroinflammation. 2016 Aug 19;13(1):188. doi: 10.1186/s12974-016-0655-y. J Neuroinflammation. 2016. PMID: 27542968 Free PMC article. - Free Sialic Acid Acts as a Signal That Promotes Streptococcus pneumoniae Invasion of Nasal Tissue and Nonhematogenous Invasion of the Central Nervous System.
Hatcher BL, Hale JY, Briles DE. Hatcher BL, et al. Infect Immun. 2016 Aug 19;84(9):2607-15. doi: 10.1128/IAI.01514-15. Print 2016 Sep. Infect Immun. 2016. PMID: 27354445 Free PMC article. - Physiology and pathophysiology of the blood-brain barrier: P-glycoprotein and occludin trafficking as therapeutic targets to optimize central nervous system drug delivery.
McCaffrey G, Davis TP. McCaffrey G, et al. J Investig Med. 2012 Dec;60(8):1131-40. doi: 10.2310/JIM.0b013e318276de79. J Investig Med. 2012. PMID: 23138008 Free PMC article. Review. - Influence of bacterial interactions on pneumococcal colonization of the nasopharynx.
Shak JR, Vidal JE, Klugman KP. Shak JR, et al. Trends Microbiol. 2013 Mar;21(3):129-35. doi: 10.1016/j.tim.2012.11.005. Epub 2012 Dec 25. Trends Microbiol. 2013. PMID: 23273566 Free PMC article. Review.
References
- Adriani K. S., van de Beek D., Brouwer M. C., Spanjaard L., de Gans J. 2007. Community-acquired recurrent bacterial meningitis in adults. Clin. Infect. Dis. 45:e46–e51 - PubMed
- Agrawal A., Simpson M. J., Black S., Carey M. P., Samols D. 2002. A C-reactive protein mutant that does not bind to phosphocholine and pneumococcal C-polysaccharide. J. Immunol. 169:3217–3222 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources