Dynamics of septin ring and collar formation in Saccharomyces cerevisiae - PubMed (original) (raw)
Dynamics of septin ring and collar formation in Saccharomyces cerevisiae
Hsin Chen et al. Biol Chem. 2011 Aug.
Abstract
Although the septin ring and collar in budding yeast were described over 20 years ago, there is still controversy regarding the organization of septin filaments within these structures and about the way in which the ring first forms and about how it converts into a collar at the mother-bud neck. Here we present quantitative analyses of the recruitment of fluorescently-tagged septins to the ring and collar through the cell cycle. Septin ring assembly began several minutes after polarity establishment and this interval was longer in daughter than in mother cells, suggesting asymmetric inheritance of septin regulators. Septins formed an initial faint and irregular ring, which became more regular as septins were recruited at a constant rate. This steady rate of septin recruitment continued for several minutes after the ring converted to a collar at bud emergence. We did not detect a stepwise change in septin fluorescence during the ring-to-collar transition. After collar formation, septins continued to accumulate at the bud neck, though at a reduced rate, until the onset of cytokinesis when the amount of neck-localized septins rapidly decreased. Implications for the mechanism of septin ring assembly are discussed.
Figures
Figure 1. Septin rings and collar through the yeast cell cycle
Cartoon showing initial small ring, hourglass collar and large split rings observed during the yeast cell cycle.
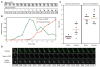
Figure 2. Timing of septin accumulation relative to the polarity marker Bem1p
(A) Illustrative cell from a deconvolved time-lapse movie of strain DLY11909 showing inverted images (so that fluorescent signal appears dark on light background) of Bem1p-GFP and Cdc3p-mCherry. (B) Quantification of Bem1p-GFP and Cdc3p-mCherry intensity for the cell in (A). (C) The interval between initial Bem1p-GFP and initial Cdc3p-mCherry detection was measured for 22 cells, separated into the 11 component mother and daughter cells (left). The interval between initial Bem1p-GFP and initial bud emergence is plotted for the same cells (right). Red lines indicate average intervals in each case. Mothers and daughters were distinguished by noting which cell displayed apical growth (tip localized Bem1p-GFP) in the previous cell cycle (daughter). (D) Illustrative mother-daughter cell. Time is indicated in min:s.
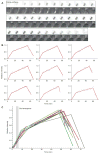
Figure 3. Kinetics of septin accumulation in the ring/collar during the cell cycle
(A) Illustrative cell from a movie of strain DLY13344 showing inverted side-on images of the septin ring/collar. DIC images show timing of bud emergence (indicated by arrow). (B) Quantification of Cdc3p-mCherry intensity in the ring/collar for a panel of nine cells. Datapoints were fit with a tri-linear scheme as described in the Methods (lines). (C) Overlay of the tri-linear fits. Gray bar indicates the time interval during which the cells underwent bud emergence.
Figure 4. Initial stages of septin ring assembly
(A) Illustrative cells from a movie of strain DLY11784 showing inverted end-on images of septin ring assembly. (B) The earliest timepoints with detectable signal were enlarged and manipulated to increase the signal and reduce the background.
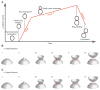
Figure 5. Summary and model for septin ring and collar formation
(A) Summary of septin recruitment during the cell cycle based on fluorescence intensity measurements. Polarity establishment, bud emergence and splitting of the septin collar at the onset of cytokinesis are indicated. (B,C) Speculative models for septin filament assembly and organization. (I) A sparse irregular ring accumulates more septins by intercalation. (II) Bud emergence and continuing septin recruitment until they form an hourglass-shaped collar (III). Slower septin recruitment continues during G2/M (IV) until the collar splits during mitotic exit (V). After cytokinesis, the remnant septin ring (VI) is of larger diameter than the new septin ring that forms in the next cycle (I).
Similar articles
- Coordinate action of distinct sequence elements localizes checkpoint kinase Hsl1 to the septin collar at the bud neck in Saccharomyces cerevisiae.
Finnigan GC, Sterling SM, Duvalyan A, Liao EN, Sargsyan A, Garcia G 3rd, Nogales E, Thorner J. Finnigan GC, et al. Mol Biol Cell. 2016 Jul 15;27(14):2213-33. doi: 10.1091/mbc.E16-03-0177. Epub 2016 May 18. Mol Biol Cell. 2016. PMID: 27193302 Free PMC article. - Architecture and dynamic remodelling of the septin cytoskeleton during the cell cycle.
Ong K, Wloka C, Okada S, Svitkina T, Bi E. Ong K, et al. Nat Commun. 2014 Dec 5;5:5698. doi: 10.1038/ncomms6698. Nat Commun. 2014. PMID: 25474997 Free PMC article. - Role of the septin ring in the asymmetric localization of proteins at the mother-bud neck in Saccharomyces cerevisiae.
Kozubowski L, Larson JR, Tatchell K. Kozubowski L, et al. Mol Biol Cell. 2005 Aug;16(8):3455-66. doi: 10.1091/mbc.e04-09-0764. Epub 2005 May 18. Mol Biol Cell. 2005. PMID: 15901837 Free PMC article. - Septins: a ring to part mother and daughter.
Faty M, Fink M, Barral Y. Faty M, et al. Curr Genet. 2002 Jun;41(3):123-31. doi: 10.1007/s00294-002-0304-0. Epub 2002 Jun 19. Curr Genet. 2002. PMID: 12111093 Review. - Septin architecture and function in budding yeast.
Farkašovský M. Farkašovský M. Biol Chem. 2020 Jul 28;401(8):903-919. doi: 10.1515/hsz-2019-0401. Biol Chem. 2020. PMID: 31913844 Review.
Cited by
- Study of impacts of two types of cellular aging on the yeast bud morphogenesis.
Tsai K, Zhou Z, Yang J, Xu Z, Xu S, Zandi R, Hao N, Chen W, Alber M. Tsai K, et al. PLoS Comput Biol. 2024 Sep 30;20(9):e1012491. doi: 10.1371/journal.pcbi.1012491. eCollection 2024 Sep. PLoS Comput Biol. 2024. PMID: 39348424 Free PMC article. - Feedback control of Swe1p degradation in the yeast morphogenesis checkpoint.
King K, Kang H, Jin M, Lew DJ. King K, et al. Mol Biol Cell. 2013 Apr;24(7):914-22. doi: 10.1091/mbc.E12-11-0812. Epub 2013 Feb 6. Mol Biol Cell. 2013. PMID: 23389636 Free PMC article. - A simple, versatile method for GFP-based super-resolution microscopy via nanobodies.
Ries J, Kaplan C, Platonova E, Eghlidi H, Ewers H. Ries J, et al. Nat Methods. 2012 Jun;9(6):582-4. doi: 10.1038/nmeth.1991. Epub 2012 Apr 29. Nat Methods. 2012. PMID: 22543348 - Role of combined cell membrane and wall mechanical properties regulated by polarity signals in cell budding.
Tsai K, Britton S, Nematbakhsh A, Zandi R, Chen W, Alber M. Tsai K, et al. Phys Biol. 2020 Oct 21;17(6):065011. doi: 10.1088/1478-3975/abb208. Phys Biol. 2020. PMID: 33085651 Free PMC article. - Analysis of Septin Reorganization at Cytokinesis Using Polarized Fluorescence Microscopy.
McQuilken M, Jentzsch MS, Verma A, Mehta SB, Oldenbourg R, Gladfelter AS. McQuilken M, et al. Front Cell Dev Biol. 2017 May 3;5:42. doi: 10.3389/fcell.2017.00042. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28516085 Free PMC article.
References
- Bobola N, Jansen RP, Shin TH, Nasmyth K. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell. 1996;84:699–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases