Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke - PubMed (original) (raw)
Clinical Trial
Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke
Jeffrey A Switzer et al. Stroke. 2011 Sep.
Abstract
Background and purpose: Plasma matrix metalloproteinase-9 levels predict posttissue plasminogen activator (tPA) hemorrhage.
Methods: The authors investigated the effect of minocycline on plasma matrix metalloproteinase-9 in acute ischemic stroke in the Minocycline to Improve Neurological Outcome in Stroke (MINOS) trial and a comparison group.
Results: Matrix metalloproteinase-9 level decreased at 72 hours compared with baseline in MINOS (tPA, P=0.0022; non-tPA, P=0.0066) and was lower than in the non-MINOS comparison group at 24 hours (tPA, P<0.0001; non-tPA, P=0.0019).
Conclusions: Lower plasma matrix metalloproteinase-9 was seen among tPA-treated subjects in the MINOS trial. Combining minocycline with tPA may prevent the adverse consequences of thrombolytic therapy through suppression of matrix metalloproteinase-9 activity.
Figures
Figure 1
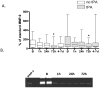
Levels of MMP-9 over time in MINOS. (A) MMP-9 levels in tPA-treated and tPA-untreated subjects at each timepoint in MINOS.Box = 25 to 75% interquartile range; horizontal line = median; vertical lines = range. *p < 0.0083. (B) A representative zymogram from a MINOS patient showing MMP-9 over time.
Figure 2
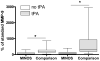
Levels of MMP-9 in MINOS and comparison subjects at 24 hours. MMP-9 in tPA-treated and tPA-untreated MINOS and comparison subjects at 24 hours. Box = 25 to 75% interquartile range; horizontal line = median; vertical lines = range. *p < 0.0125.
Comment in
- Letter by Lee regarding article, "Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute stroke".
Lee R. Lee R. Stroke. 2011 Oct;42(10):e566; author reply e567. doi: 10.1161/STROKEAHA.111.632869. Epub 2011 Sep 8. Stroke. 2011. PMID: 21903959 No abstract available.
Similar articles
- Extension of the thrombolytic time window with minocycline in experimental stroke.
Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Murata Y, et al. Stroke. 2008 Dec;39(12):3372-7. doi: 10.1161/STROKEAHA.108.514026. Epub 2008 Oct 16. Stroke. 2008. PMID: 18927459 Free PMC article. - Letter by Lee regarding article, "Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute stroke".
Lee R. Lee R. Stroke. 2011 Oct;42(10):e566; author reply e567. doi: 10.1161/STROKEAHA.111.632869. Epub 2011 Sep 8. Stroke. 2011. PMID: 21903959 No abstract available. - Effects of minocycline plus tissue plasminogen activator combination therapy after focal embolic stroke in type 1 diabetic rats.
Fan X, Lo EH, Wang X. Fan X, et al. Stroke. 2013 Mar;44(3):745-52. doi: 10.1161/STROKEAHA.111.000309. Epub 2013 Feb 19. Stroke. 2013. PMID: 23422086 Free PMC article. - Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline.
Hess DC, Fagan SC. Hess DC, et al. Pharmacotherapy. 2010 Jul;30(7 Pt 2):55S-61S. doi: 10.1592/phco.30.pt2.55S. Pharmacotherapy. 2010. PMID: 20575623 Free PMC article. Review. - Low-Dose Tissue Plasminogen Activator in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis.
Cheng JW, Zhang XJ, Cheng LS, Li GY, Zhang LJ, Ji KX, Zhao Q, Bai Y. Cheng JW, et al. J Stroke Cerebrovasc Dis. 2018 Feb;27(2):381-390. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.014. Epub 2017 Oct 27. J Stroke Cerebrovasc Dis. 2018. PMID: 29111341 Review.
Cited by
- Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia.
Cui J, Chen S, Zhang C, Meng F, Wu W, Hu R, Hadass O, Lehmidi T, Blair GJ, Lee M, Chang M, Mobashery S, Sun GY, Gu Z. Cui J, et al. Mol Neurodegener. 2012 May 15;7:21. doi: 10.1186/1750-1326-7-21. Mol Neurodegener. 2012. PMID: 22587708 Free PMC article. - Minocycline prevents IL-6 increase after acute ischemic stroke.
Switzer JA, Sikora A, Ergul A, Waller JL, Hess DC, Fagan SC. Switzer JA, et al. Transl Stroke Res. 2012 Sep;3(3):363-8. doi: 10.1007/s12975-012-0150-4. Epub 2012 Mar 15. Transl Stroke Res. 2012. PMID: 23105953 Free PMC article. - The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma.
Fields GB. Fields GB. Cells. 2019 Aug 27;8(9):984. doi: 10.3390/cells8090984. Cells. 2019. PMID: 31461880 Free PMC article. Review. - Laminin as a Biomarker of Blood-Brain Barrier Disruption under Neuroinflammation: A Systematic Review.
Zapata-Acevedo JF, García-Pérez V, Cabezas-Pérez R, Losada-Barragán M, Vargas-Sánchez K, González-Reyes RE. Zapata-Acevedo JF, et al. Int J Mol Sci. 2022 Jun 17;23(12):6788. doi: 10.3390/ijms23126788. Int J Mol Sci. 2022. PMID: 35743229 Free PMC article. Review. - Neuroprotective Therapies for Spontaneous Intracerebral Hemorrhage.
Kearns KN, Ironside N, Park MS, Worrall BB, Southerland AM, Chen CJ, Ding D. Kearns KN, et al. Neurocrit Care. 2021 Dec;35(3):862-886. doi: 10.1007/s12028-021-01311-3. Epub 2021 Aug 2. Neurocrit Care. 2021. PMID: 34341912 Review.
References
- Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, Wang X, Zhu M, Sorensen AG, Lo EH, Kelly PJ. Association between tpa therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. - PubMed
- Montaner J, Alvarez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J, Gonzalez MA, Monasterio J. Matrix metalloproteinase expression after human cardioembolic stroke: Temporal profile and relation to neurological impairment. Stroke. 2001;32:1759–1766. - PubMed
- Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, Quintana M, Alvarez-Sabin J. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS055728-03/NS/NINDS NIH HHS/United States
- R01 NS055728/NS/NINDS NIH HHS/United States
- R01 NS063965/NS/NINDS NIH HHS/United States
- NS063965/NS/NINDS NIH HHS/United States
- R01NS055728/NS/NINDS NIH HHS/United States
- R01 NS055728-01A1/NS/NINDS NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- I01 BX000347/BX/BLRD VA/United States
- R01 NS055728-02/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical