Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent - PubMed (original) (raw)
Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent
Crissey L Pascale et al. Fluids Barriers CNS. 2011.
Abstract
Background: Age is the major risk factor for many neurodegenerative diseases, including Alzheimer's disease (AD). There is an accumulation of amyloid-beta peptides (Aβ) in both the AD brain and the normal aging brain. Clearance of Aβ from the brain occurs via active transport at the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB). With increasing age, the expression of the Aβ efflux transporters is decreased and the Aβ influx transporter expression is increased at the BBB, adding to the amyloid burden in the brain. Expression of the Aβ transporters at the choroid plexus (CP) epithelium as a function of aging was the subject of this study.
Methods: This project investigated the changes in expression of the Aβ transporters, the low density lipoprotein receptor-related protein-1 (LRP-1), P-glycoprotein (P-gp), LRP-2 (megalin) and the receptor for advanced glycation end-products (RAGE) at the BCSFB in Brown-Norway/Fischer rats at ages 3, 6, 9, 12, 20, 30 and 36 months, using real time RT-PCR to measure transporter mRNA expression, and immunohistochemistry (IHC) to measure transporter protein in isolated rat CP.
Results: There was an increase in the transcription of the Aβ efflux transporters, LRP-1 and P-gp, no change in RAGE expression and a decrease in LRP-2, the CP epithelium influx transporter, at the BCSFB with aging. Decreased Aβ42 concentration in the CP, as measured by quantitative IHC, was associated with these Aβ transporter alterations.
Conclusions: Age-dependent alterations in the CP Aβ transporters are associated with a decrease in Aβ42 accumulation in the CP, and are reciprocal to the changes seen in these transporters at the BBB, suggesting a possible compensatory role for the BCSFB in Aβ clearance in aging.
Figures
Figure 1
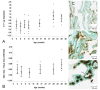
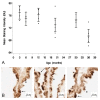
LRP-1 expression at the CP epithelium with age. (A) Graph of the log-transformed normalized expression of LRP-1 with age, n = 8 for each age group tested (4 rats, 8 CPs pooled for each "n", 32 rats for an n = 8). One-way ANOVA revealed a significant increase in LRP-1 expression (p = 0.047). Significance was reached between 9 and 30 mo 95% confidence limits (0.0122, 1.1828). Error bars represent 95% confidence intervals for the means_. (_B) Semi-quantitative IHC for LRP-1 expression in grayscale units (GU). Mean staining intensity was significantly different (_p <_0.05) for the six age groups, n = 5 for each age group (5 rats, 2 CPs for each sample). (C) IHC of LRP-1 expression at 3 mo (top), 30 mo (center) and 36 mo (bottom) old rats. Staining is localized to the apical membrane (arrows).
Figure 2
Expression of LRP-2 at the CP with age. (A) Graph of the log-transformed normalized expression of LRP-2 with respect to age, n = 8 per age group. One-way ANOVA revealed a significant decrease (p = 0.0005) with age on LRP-2 expression. Significance was reached between 3 and 9, 12, 20, 30, and 36 mo. Error bars represent 95% confidence intervals for the means. (B) Semi-quantitative IHC for LRP-2 expression in grayscale units (GU). Mean staining intensity was significantly decreased (_p <_0.05) for the age groups, n = 5 per age group. (C) IHC of LRP-2 expression at 3 mo (top), 20 mo (center), and 36 mo (bottom) old rats. Staining is localized to the apical membrane (arrows), and is also seen subapically.
Figure 3
Age-related P-gp expression at the CP. (A) Graph of the log-transformed normalized expression of P-gp with respect to age, n = 8 for each age group. One-way ANOVA revealed a significant increase (p = 0.0005) with age on P-gp expression. Significance was reached between 30 mo and 3, 6, 9, and 12 mo, and between 36 mo and 6 mo. Error bars represent 95% confidence intervals for the means. (B) Semi-quantitative IHC for P-gp expression. Stain area to tissue area ratio was not significantly different (p > 0.05) for the six age groups, n = 5 per age group. (C) IHC of P-gp expression at 3 mo (top), 20 mo (center), and 36 mo (bottom) old rats. Majority of staining is localized to the basolateral membrane (arrows), though on occasion it is seen along the apical membrane (asterisk).
Figure 4
Aβ42 concentration in the CP epithelium with age. (A) Semi-quantitative IHC for Aβ42 deposition. Mean staining intensity in grayscale units (GU) was significantly decreased (p < 0.05) for the age groups, n = 5 per age group. (B) IHC of Aβ42 at 3 mo (left), 20 mo (center), and 36 mo (right) old rats. Staining is granular and primarily cytosolic (asterisks), though also found along the apical membrane (arrows).
Figure 5
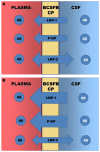
Diagrams of the direction and expression of the Aβ transporters at the BCSFB. (A) Diagram to show the direction and relative expression of LRP-1, P-gp and LRP-2 at three months of age in the BN/F rat. (B) Diagram of the relative expression of the three transporters after 20 months. Note that LRP-1 and P-gp expression increase, whereas LRP-2 expression decreases with age.
Similar articles
- Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging.
Silverberg GD, Messier AA, Miller MC, Machan JT, Majmudar SS, Stopa EG, Donahue JE, Johanson CE. Silverberg GD, et al. J Neuropathol Exp Neurol. 2010 Oct;69(10):1034-43. doi: 10.1097/NEN.0b013e3181f46e25. J Neuropathol Exp Neurol. 2010. PMID: 20838242 - Age-Dependent Regulation of the Blood-Brain Barrier Influx/Efflux Equilibrium of Amyloid-β Peptide in a Mouse Model of Alzheimer's Disease (3xTg-AD).
Do TM, Dodacki A, Alata W, Calon F, Nicolic S, Scherrmann JM, Farinotti R, Bourasset F. Do TM, et al. J Alzheimers Dis. 2016;49(2):287-300. doi: 10.3233/JAD-150350. J Alzheimers Dis. 2016. PMID: 26484906 - Clearance of amyloid-β peptide across the choroid plexus in Alzheimer's disease.
Alvira-Botero X, Carro EM. Alvira-Botero X, et al. Curr Aging Sci. 2010 Dec;3(3):219-29. doi: 10.2174/1874609811003030219. Curr Aging Sci. 2010. PMID: 20735345 Review. - Transporters in the brain endothelial barrier.
Ueno M, Nakagawa T, Wu B, Onodera M, Huang CL, Kusaka T, Araki N, Sakamoto H. Ueno M, et al. Curr Med Chem. 2010;17(12):1125-38. doi: 10.2174/092986710790827816. Curr Med Chem. 2010. PMID: 20175745 Review.
Cited by
- Engineered Antibodies to Improve Efficacy against Neurodegenerative Disorders.
Niazi SK, Mariam Z, Magoola M. Niazi SK, et al. Int J Mol Sci. 2024 Jun 18;25(12):6683. doi: 10.3390/ijms25126683. Int J Mol Sci. 2024. PMID: 38928395 Free PMC article. Review. - Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months.
Chiu C, Miller MC, Caralopoulos IN, Worden MS, Brinker T, Gordon ZN, Johanson CE, Silverberg GD. Chiu C, et al. Fluids Barriers CNS. 2012 Jan 23;9(1):3. doi: 10.1186/2045-8118-9-3. Fluids Barriers CNS. 2012. PMID: 22269091 Free PMC article. - Memory Impairment in Estrogen Receptor α Knockout Mice Through Accumulation of Amyloid-β Peptides.
Hwang CJ, Yun HM, Park KR, Song JK, Seo HO, Hyun BK, Choi DY, Yoo HS, Oh KW, Hwang DY, Han SB, Hong JT. Hwang CJ, et al. Mol Neurobiol. 2015 Aug;52(1):176-86. doi: 10.1007/s12035-014-8853-z. Epub 2014 Aug 17. Mol Neurobiol. 2015. PMID: 25128029 Free PMC article. - Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier.
Lim DC, Pack AI. Lim DC, et al. Sleep Med Rev. 2014 Feb;18(1):35-48. doi: 10.1016/j.smrv.2012.12.003. Epub 2013 Mar 28. Sleep Med Rev. 2014. PMID: 23541562 Free PMC article. Review. - Clearance systems in the brain-implications for Alzheimer disease.
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Tarasoff-Conway JM, et al. Nat Rev Neurol. 2015 Aug;11(8):457-70. doi: 10.1038/nrneurol.2015.119. Epub 2015 Jul 21. Nat Rev Neurol. 2015. PMID: 26195256 Free PMC article. Review.
References
- Selkoe DJ. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann NY Acad Sci. 2000;924:17–25. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous