CaMKII in the cardiovascular system: sensing redox states - PubMed (original) (raw)
Review
CaMKII in the cardiovascular system: sensing redox states
Jeffrey R Erickson et al. Physiol Rev. 2011 Jul.
Abstract
The multifunctional Ca(2+)- and calmodulin-dependent protein kinase II (CaMKII) is now recognized to play a central role in pathological events in the cardiovascular system. CaMKII has diverse downstream targets that promote vascular disease, heart failure, and arrhythmias, so improved understanding of CaMKII signaling has the potential to lead to new therapies for cardiovascular disease. CaMKII is a multimeric serine-threonine kinase that is initially activated by binding calcified calmodulin (Ca(2+)/CaM). Under conditions of sustained exposure to elevated Ca(2+)/CaM, CaMKII transitions into a Ca(2+)/CaM-autonomous enzyme by two distinct but parallel processes. Autophosphorylation of threonine-287 in the CaMKII regulatory domain "traps" CaMKII into an open configuration even after Ca(2+)/CaM unbinding. More recently, our group identified a pair of methionines (281/282) in the CaMKII regulatory domain that undergo a partially reversible oxidation which, like autophosphorylation, prevents CaMKII from inactivating after Ca(2+)/CaM unbinding. Here we review roles of CaMKII in cardiovascular disease with an eye to understanding how CaMKII may act as a transduction signal to connect pro-oxidant conditions into specific downstream pathological effects that are relevant to rare and common forms of cardiovascular disease.
Figures
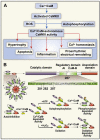
Figure 1
Oxidation and autophosphorylation both convert CaMKII into a Ca2+/CaM-independent enzyme by modification of defined CaMKII regulatory domain amino acids.
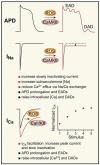
Figure 2
ROS and CaMKII both increase the slowly inactivating component of INa and enhance ICa facilitation, leading to action potential duration (APD) prolongation and early (EADs) and delayed (DADs) afterdepolarizations.
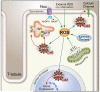
Figure 3
Reactive oxygen species and CaMKII in cardiomyocytes.
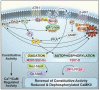
Figure 4
CaMKII is a likely participant in complex intercellular crosstalk between calcium and ROS signaling mechanisms.
Figure 5
The oxidation state of CaMKII is acutely sensitive to balance between ROS producing and ROS ablating processes
Figure 6
Mechanical and biological factors increase the production of ROS and activate CaMKII in vascular smooth muscle cells, resulting in impaired vessel tone, enhanced inflammatory response, and increased SMC migration, proliferation, and apoptosis.
Similar articles
- A Memory Molecule, Ca(2+)/Calmodulin-Dependent Protein Kinase II and Redox Stress; Key Factors for Arrhythmias in a Diseased Heart.
Song YH. Song YH. Korean Circ J. 2013 Mar;43(3):145-51. doi: 10.4070/kcj.2013.43.3.145. Korean Circ J. 2013. PMID: 23613689 Free PMC article. - Interactive Roles of CaMKII/Ryanodine Receptor Signaling and Inflammation in Lung Diseases.
Wang L, Ginnan RG, Wang YX, Zheng YM. Wang L, et al. Adv Exp Med Biol. 2021;1303:305-317. doi: 10.1007/978-3-030-63046-1_16. Adv Exp Med Biol. 2021. PMID: 33788199 Review. - The KN-93 Molecule Inhibits Calcium/Calmodulin-Dependent Protein Kinase II (CaMKII) Activity by Binding to Ca2+/CaM.
Wong MH, Samal AB, Lee M, Vlach J, Novikov N, Niedziela-Majka A, Feng JY, Koltun DO, Brendza KM, Kwon HJ, Schultz BE, Sakowicz R, Saad JS, Papalia GA. Wong MH, et al. J Mol Biol. 2019 Mar 29;431(7):1440-1459. doi: 10.1016/j.jmb.2019.02.001. Epub 2019 Feb 10. J Mol Biol. 2019. PMID: 30753871 - CaMKII oxidative activation and the pathogenesis of cardiac disease.
Luczak ED, Anderson ME. Luczak ED, et al. J Mol Cell Cardiol. 2014 Aug;73:112-6. doi: 10.1016/j.yjmcc.2014.02.004. Epub 2014 Feb 13. J Mol Cell Cardiol. 2014. PMID: 24530899 Free PMC article. Review. - A significant but rather mild contribution of T286 autophosphorylation to Ca2+/CaM-stimulated CaMKII activity.
Coultrap SJ, Barcomb K, Bayer KU. Coultrap SJ, et al. PLoS One. 2012;7(5):e37176. doi: 10.1371/journal.pone.0037176. Epub 2012 May 16. PLoS One. 2012. PMID: 22615928 Free PMC article.
Cited by
- Folic acid reverses nitric oxide synthase uncoupling and prevents cardiac dysfunction in insulin resistance: role of Ca2+/calmodulin-activated protein kinase II.
Roe ND, He EY, Wu Z, Ren J. Roe ND, et al. Free Radic Biol Med. 2013 Dec;65:234-243. doi: 10.1016/j.freeradbiomed.2013.06.042. Epub 2013 Jun 29. Free Radic Biol Med. 2013. PMID: 23820268 Free PMC article. - Temporal pattern of neuronal insulin release during Caenorhabditis elegans aging: Role of redox homeostasis.
Minniti AN, Arriagada H, Zúñiga S, Bravo-Zehnder M, Alfaro IE, Aldunate R. Minniti AN, et al. Aging Cell. 2019 Feb;18(1):e12855. doi: 10.1111/acel.12855. Epub 2018 Nov 19. Aging Cell. 2019. PMID: 30456853 Free PMC article. - Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors.
Sausville E, Lorusso P, Carducci M, Carter J, Quinn MF, Malburg L, Azad N, Cosgrove D, Knight R, Barker P, Zabludoff S, Agbo F, Oakes P, Senderowicz A. Sausville E, et al. Cancer Chemother Pharmacol. 2014 Mar;73(3):539-49. doi: 10.1007/s00280-014-2380-5. Epub 2014 Jan 22. Cancer Chemother Pharmacol. 2014. PMID: 24448638 Free PMC article. Clinical Trial. - The cGMP/PKG pathway as a common mediator of cardioprotection: translatability and mechanism.
Inserte J, Garcia-Dorado D. Inserte J, et al. Br J Pharmacol. 2015 Apr;172(8):1996-2009. doi: 10.1111/bph.12959. Epub 2015 Mar 16. Br J Pharmacol. 2015. PMID: 25297462 Free PMC article. Review. - Ca2+/calmodulin-dependent protein kinase II function in vascular remodelling.
Singer HA. Singer HA. J Physiol. 2012 Mar 15;590(6):1349-56. doi: 10.1113/jphysiol.2011.222232. Epub 2011 Nov 28. J Physiol. 2012. PMID: 22124148 Free PMC article. Review.
References
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. - PubMed
- The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. - PubMed
- MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. - PubMed
- Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S, Denney T, Jr., Powell P, McGiffin DC, Dell’Italia LJ. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60% J Am Coll Cardiol. 2010;55:671–679. - PMC - PubMed
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL086350/HL/NHLBI NIH HHS/United States
- R01-HL-079031/HL/NHLBI NIH HHS/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- T32-HL-86350/HL/NHLBI NIH HHS/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
- R01-HL-62494/HL/NHLBI NIH HHS/United States
- R01-HL-70250/HL/NHLBI NIH HHS/United States
- R01 HL062494/HL/NHLBI NIH HHS/United States
- T32 GM067795/GM/NIGMS NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous