The Impact of Preeclampsia on Gene Expression at the Maternal-Fetal Interface - PubMed (original) (raw)
The Impact of Preeclampsia on Gene Expression at the Maternal-Fetal Interface
Virginia D Winn et al. Pregnancy Hypertens. 2011.
Abstract
Preeclampsia (PE) impacts 8 million mother-infant pairs worldwide each year. This human pregnancy-specific disease characterized by hypertension and proteinuria accounts for significant maternal and neonatal morbidity and mortality. The current theory of the pathogenesis of PE as reviewed by Drs. Christopher Redman and Ian Sargent is thought to occur as a 2-stage process with poor placentation in the first half of pregnancy resulting in the maternal response in the second half of pregnancy. Our studies have focused on understanding the placental contribution to this serious disease by examining the gene expression profile of the deciduas basalis or basal plate, the region of the placenta involved in the "poor placentation". In this review we present summaries of our microarray datasets both of normal placentation and those gene expression changes resulting in the context of PE. Additionally, we have taken this opportunity to combine the data sets to provide a more comprehensive view of this region of the placenta. As defects in the basal plate are, in part, at the root of the disease process, we believe that understanding the pathobiology that occurs in this region will increase our ability to alter the development and/or course of PE.
Figures
Figure 1. Diagram of the human maternal-fetal interface
(A) Representation of the human placenta after delivery. The placental surface that was adjacent to the uterine wall is termed the basal plate. The boxed area denotes the region biopsied for these studies. (B) View of the basal plate at the cellular level. This chimeric region of the placenta is composed of both maternal and fetal cells: extravillous (invasive) cytotrophoblasts (dark grey), decidual cells (light grey), remodeled vasculature (both invasive CTBs and maternal endothelium) and maternal immune cells (white). (Reproduced with permission from Endocrinology (13)).
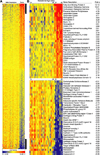
Figure 2. Heat map of the most highly up regulated (upper panel) and down regulated (lower panel) differentially expressed genes in the basal plate region at term in normal pregnancy
The normalized log intensity values were centered to the median value of each probe set and colored on a range of −2 to +2. Red denotes up regulated, yellow denotes intermediate, and blue denotes down regulated expression levels as compared with the median value. Columns contain data from a single basal plate specimen, and rows correspond to a single probe set. Samples are arranged from left to right, ordered by increasing gestational age. Rows are ranked by fold change (mean term value [n = 9] divided by mean midgestation value [n = 27]). (Reproduced with permission from Endocrinology (13))
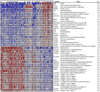
Figure 3. Heat map of differentially expressed genes in basal plates of PE placentas as compared to controls
The normalized log intensity values for 71 differentially expressed probe sets were centered to the median value of each probe set and colored on a range of −2.5 to +2.5. Red denotes upregulated and blue denotes downregulated expression levels as compared with the median value. Columns contain data from a single basal plate specimen, and rows correspond to a single probe set. Samples are arranged from left to right, ordered by increasing gestational age within each category. Rows are ranked by fold change (mean PE value [n = 12] divided by mean PTL value [n = 11]). (Reproduced with permission from Endocrinology (42)).
Figure 4. Gestational Timeline of Basal Plate Biopsies Used in the Composite Analysis
The microarray data from human basal plate biopsies were used in a combined analysis. Each black box represents one individual placenta and is listed by gestational age and condition.
Figure 5. Heat map of the most highly up regulated and down regulated differentially expressed genes in basal plates of PE placentas as compared to the second trimester, term and preterm labor samples
The normalized log intensity values for the differentially expressed probe sets were centered to the median value of each probe set and colored on a range of −2.5 to +2.5. Red denotes up regulated and blue denotes down regulated expression levels as compared with the median value. Columns contain data from a single basal plate specimen, and rows correspond to a single probe set. Samples within each catergory are arranged from left to right, ordered by increasing gestational age. Rows are ranked by fold change. The complete heat map is shown in supplemental Fig. S1.
Similar articles
- Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat.
Barrientos G, Pussetto M, Rose M, Staff AC, Blois SM, Toblli JE. Barrientos G, et al. Mol Hum Reprod. 2017 Jul 1;23(7):509-519. doi: 10.1093/molehr/gax024. Mol Hum Reprod. 2017. PMID: 28402512 - Pathogenesis of Preeclampsia and Therapeutic Approaches Targeting the Placenta.
Jena MK, Sharma NR, Petitt M, Maulik D, Nayak NR. Jena MK, et al. Biomolecules. 2020 Jun 24;10(6):953. doi: 10.3390/biom10060953. Biomolecules. 2020. PMID: 32599856 Free PMC article. Review. - Pathophysiology of placentation abnormalities in pregnancy-induced hypertension.
Furuya M, Ishida J, Aoki I, Fukamizu A. Furuya M, et al. Vasc Health Risk Manag. 2008;4(6):1301-13. doi: 10.2147/vhrm.s4009. Vasc Health Risk Manag. 2008. PMID: 19337544 Free PMC article. Review. - Management of preeclampsia.
Dekker GA. Dekker GA. Pregnancy Hypertens. 2014 Jul;4(3):246-7. doi: 10.1016/j.preghy.2014.04.021. Epub 2014 Jul 9. Pregnancy Hypertens. 2014. PMID: 26104648 - Placental C4d deposition is a feature of defective placentation: observations in cases of preeclampsia and miscarriage.
Kim EN, Yoon BH, Lee JY, Hwang D, Kim KC, Lee J, Shim JY, Kim CJ. Kim EN, et al. Virchows Arch. 2015 Jun;466(6):717-25. doi: 10.1007/s00428-015-1759-y. Epub 2015 Mar 28. Virchows Arch. 2015. PMID: 25820373
Cited by
- Laminins Regulate Placentation and Pre-eclampsia: Focus on Trophoblasts and Endothelial Cells.
Liu M, Yin Y, Yu H, Zhou R. Liu M, et al. Front Cell Dev Biol. 2020 Aug 7;8:754. doi: 10.3389/fcell.2020.00754. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850857 Free PMC article. Review. - Analysis of the placental tissue transcriptome of normal and preeclampsia complicated pregnancies.
Trifonova EA, Gabidulina TV, Ershov NI, Serebrova VN, Vorozhishcheva AY, Stepanov VA. Trifonova EA, et al. Acta Naturae. 2014 Apr;6(2):71-83. Acta Naturae. 2014. PMID: 25093114 Free PMC article. - Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology.
Garrido-Gomez T, Dominguez F, Quiñonero A, Diaz-Gimeno P, Kapidzic M, Gormley M, Ona K, Padilla-Iserte P, McMaster M, Genbacev O, Perales A, Fisher SJ, Simón C. Garrido-Gomez T, et al. Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):E8468-E8477. doi: 10.1073/pnas.1706546114. Epub 2017 Sep 18. Proc Natl Acad Sci U S A. 2017. PMID: 28923940 Free PMC article. - Early detection of maternal risk for preeclampsia.
Mikat B, Gellhaus A, Wagner N, Birdir C, Kimmig R, Köninger A. Mikat B, et al. ISRN Obstet Gynecol. 2012;2012:172808. doi: 10.5402/2012/172808. Epub 2012 Jul 17. ISRN Obstet Gynecol. 2012. PMID: 22852092 Free PMC article. - MMP-9 and TIMP-1 in placenta of hypertensive disorder complicating pregnancy.
Zhang Y, Li P, Guo Y, Liu X, Zhang Y. Zhang Y, et al. Exp Ther Med. 2019 Jul;18(1):637-641. doi: 10.3892/etm.2019.7591. Epub 2019 May 17. Exp Ther Med. 2019. PMID: 31258700 Free PMC article.
References
- Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, et al. Integrin switching regulates normal trophoblast invasion. Development. 1994 Dec;120(12):3657–3666. - PubMed
- Clark DE, Smith SK, Licence D, Evans AL, Charnock-Jones DS. Comparison of expression patterns for placenta growth factor, vascular endothelial growth factor (VEGF), VEGF-B and VEGF-C in the human placenta throughout gestation. J Endocrinol. 1998 Dec;159(3):459–467. - PubMed
- Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002 Apr;160(4):1405–1423. - PMC - PubMed
Grants and funding
- R01 HL064597-01/HL/NHLBI NIH HHS/United States
- K12 HD001271-05/HD/NICHD NIH HHS/United States
- R01 HL064597/HL/NHLBI NIH HHS/United States
- R01 HD060723/HD/NICHD NIH HHS/United States
- R01 HL072301-01/HL/NHLBI NIH HHS/United States
- P01 HD030367/HD/NICHD NIH HHS/United States
- K12 HD000849/HD/NICHD NIH HHS/United States
- R01 HD060723-01A1/HD/NICHD NIH HHS/United States
- P01 HD030367-06/HD/NICHD NIH HHS/United States
- R01 HL072301/HL/NHLBI NIH HHS/United States
- K12 HD000849-12/HD/NICHD NIH HHS/United States
- M01 RR000833-25/RR/NCRR NIH HHS/United States
- K12 HD001271/HD/NICHD NIH HHS/United States
- M01 RR000083/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials