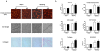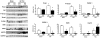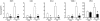H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of STAT3 and accelerates the transition into heart failure on cardiac overload - PubMed (original) (raw)
. 2011 Jul 26;124(4):406-15.
doi: 10.1161/CIRCULATIONAHA.110.013847. Epub 2011 Jul 11.
Paulo Lizano, Lydie Laure, Xiangzhen Sui, Eman Rashed, Ji Yeon Park, Chull Hong, Shumin Gao, Eric Holle, Didier Morin, Sunil K Dhar, Thomas Wagner, Alain Berdeaux, Bin Tian, Stephen F Vatner, Christophe Depre
Affiliations
- PMID: 21747053
- PMCID: PMC3369833
- DOI: 10.1161/CIRCULATIONAHA.110.013847
H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of STAT3 and accelerates the transition into heart failure on cardiac overload
Hongyu Qiu et al. Circulation. 2011.
Abstract
Background: Cardiac overload, a major cause of heart failure, induces the expression of the heat shock protein H11 kinase/Hsp22 (Hsp22).
Methods and results: To determine the specific function of Hsp22 in that context, a knockout mouse model of Hsp22 deletion was generated. Although comparable to wild-type mice in basal conditions, knockout mice exposed to pressure overload developed less hypertrophy and showed ventricular dilation, impaired contractile function, increased myocyte length and accumulation of interstitial collagen, faster transition into heart failure, and increased mortality. Microarrays revealed that hearts from knockout mice failed to transactivate genes regulated by the transcription factor STAT3. Accordingly, nuclear STAT3 tyrosine phosphorylation was decreased in knockout mice. Silencing and overexpression experiments in isolated neonatal rat cardiomyocytes showed that Hsp22 activates STAT3 via production of interleukin-6 by the transcription factor nuclear factor-κB. In addition to its transcriptional function, STAT3 translocates to the mitochondria where it increases oxidative phosphorylation. Both mitochondrial STAT3 translocation and respiration were also significantly decreased in knockout mice.
Conclusions: This study found that Hsp22 represents a previously undescribed activator of both nuclear and mitochondrial functions of STAT3, and its deletion in the context of pressure overload in vivo accelerates the transition into heart failure and increases mortality.
Figures
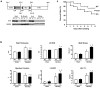
Figure 1. Characteristics of the KO mouse submitted to pressure overload
A. Generation of the Hsp22 KO mouse. Construct design (the gray box is exon 1) and Hsp22 expression in WT, heterozygous and KO mice, in various tissues. B. Functional characteristics of WT and KO mice (n=6 per group) in basal conditions (sham) and after two weeks TAC. The graphs show the LV septal wall thickness, end-diastolic diameter (EDD), wall stress, ejection fraction, end-diastolic pressure (EDP) and lung weight/tibial length (LW/TL). C. Survival curve of WT and KO mice after TAC and followed up to two weeks (n≥20 per group; there was no additional mortality in either group after day 7). *, P<0.05 versus corresponding sham; #, P<0.05 versus corresponding WT (2-by-2 analysis).
Figure 2
Morphological characteristics of WT and KO mice (n=6–8 per group) in basal conditions (sham) and after two weeks TAC. A. Representative examples of cross-sectional area, myocyte length and interstitial collagen accumulation (PSR staining). B. Quantitation of LV/TL, myocytes cross-sectional area, cell length and volume measured after myocytes isolation, and collagen accumulation and apoptosis (measured in all cell types) reported per myocardial surface. *, P<0.05 versus corresponding sham; #, P<0.05 versus corresponding WT (2-by-2 analysis).
Figure 3. Hsp22 deletion impairs the activation of stress-responsive pathways
Immunoblotting for P-Akt, Akt, P-Smad 1/5/8, Smad 1, P-ERK 1, P-ERK 2, ERK 1/2, iNOS and Hsp70 in WT versus KO, both in sham conditions and after three days TAC. Bar graphs for n=5 per group. GAPDH is used as a loading control. *, P<0.05 versus corresponding sham; #, P<0.05 versus corresponding WT (2-by-2 analysis).
Figure 4. Microarray analysis of WT and KO mice
A. Number of genes differentially regulated between KO versus WT in sham, and after 3 days and 2 weeks TAC. B. Venn diagram showing differences of regulated genes between KO and WT in sham and 3 days TAC. C. Gene ontology categories, corresponding to genes with lower expression in KO versus WT 3 days after TAC, are indicated. The gene density map reflects distribution of genes, with color corresponding to the scale shown in the Figure. For example, “+40%” and “−40%”, shown as red and blue indicate 40% enrichment and 40% depletion, respectively. D. Expression changes of STAT3-regulated genes in Hsp22 KO 3 days after TAC. STAT3-annotated genes were compared to other genes on the microarray using the cumulative distribution function (CDF) based on log2 ratio (fold change). The black dotted curve is for STAT3-regulated genes, and the grey curve for other genes. For each curve, the fraction of genes at which log2(ratio)=0 is indicated. The P value indicating the difference between both curves is calculated from the Kolmogorov-Smirnov test.
Figure 5. Validation of the microarray experiments by quantitative PCR
RNA expression of STAT3-regulated genes in hearts from WT versus KO mouse, in sham conditions or three days after TAC. Results are normalized per cyclophilin (CPH) transcript. *, P<0.05 versus corresponding sham; #, P<0.05 versus corresponding WT (2-by-2 analysis). Abbreviations: EGR-1, early growth response-1; CISH, cytokine-inducible SH2-containing protein; Bcl3, B cell leukemia/lymphoma-3; THBS-1, thrombospondin-1; NPC-1, Niemann Pick type C1.
Figure 6. Regulation of STAT by Hsp22
A. Subcellular fractionation, using markers for cytosol (cyt), microsomes (mic), mitochondria (mit) and nuclei (nuc). B. Expression of Hsp22, STAT3, Y705 P-STAT3, STAT1 and Y701 P-STAT1 in nuclear fractions from Hsp22 TG or KO mice and respective WT littermates. Lamin A/C is used as a loading control. Bar graphs for n=3 per group. *, P<0.05 versus corresponding WT (2-by-2 analysis). C. Expression of Hsp22, STAT3 and S727 P-STAT3 in mitochondrial fractions from Hsp22 TG or KO mice and respective WT littermates. The NADH dehydrogenase 1 alpha subunit 9 (NDUFA9) is used as a loading control. Bar graphs represent the mean±SEM for n=3 per group. *, P<0.05 versus corresponding WT (2-by-2 analysis). D. Treatment of mitochondrial fractions with 2% digitonin followed by centrifugation. Porin, used as a marker of outer mitochondrial membrane, is released into the supernatant upon addition of digitonin, whereas complex III (Co. III), a marker of the mitoplast, remains in the undigested pellet, together with both STAT3 and Hsp22. E. Immunoprecipitation of STAT3 in mitochondrial fractions followed by western blotting for both Hsp22 and STAT3 in a heart sample from both WT and TG mice. The upper band for Hsp22 in the TG sample corresponds to the hemagglutinin-tagged transgenic product. Repeated immunoprecipitation (Re-IP) on a TG protein sample refers to a second incubation of the protein sample with the same antibody. Re-IP should not provide any band upon immunoblotting if the protein of interest has been successfully depleted by the first immunoprecipitation. F. Measurement of mitochondrial respiration rate (states 2, 3 and 4), state 3/4 respiration coefficient ratio (RCR), ADP/O ratio and maximal respiration under uncoupling by FCCP in Hsp22 TG or KO mice and respective WT littermates (n=5 per group). *, P<0.05 versus corresponding WT (one-way ANOVA).
Figure 7. Hsp22-mediated activation of STAT3 by NF-κB
A. Impact of Hsp22 over-expression (5 to 25 moi) on tyrosine phosphorylation of STAT1 and STAT3 in isolated cardiac myocytes, and on STAT DNA binding activity in presence or in absence of SN50 (n=3 per group). *, P<0.05 versus LacZ control (two-way ANOVA with 2 factors). B. Immunofluorescence for Y705 P-STAT3 (with or without superimposition of nuclear DAPI) and for Hsp22 in cardiac myocytes infected with the Hsp22 adenovirus in presence or in absence of SN50, and compared to a LacZ control. C. Expression of gp130, CT-1, LIF and IL-6 in response to Hsp22 over-expression, response of IL-6 to Hsp22 over-expression in presence of SN50, IL-6 release (as measured by ELISA) in the extracellular medium of isolated neonatal rat cardiac myocytes (n=3 per group) in response to different moi of Hsp22 adenovirus, and DNA binding activity of NF-κB in response to Hsp22 over-expression (20 moi) as compared to LacZ control (n= 3 per group). **, P<0.01; *, P<0.05 versus LacZ control (one-way ANOVA with four factor levels). D. Impact of Hsp22 silencing on STAT3 phosphorylation in response to IL-1β, LIF and IL-6 stimulation in isolated cardiac myocytes infected with a hairpin harboring a sequence against luciferase (Luc) as a control or against Hsp22 (si). n=4 per group. **, P<0.01 versus corresponding vehicle; #, P<0.05 versus corresponding Luc control (two-way ANOVA). E. Measurement of plasma IL-6 concentration in TG and KO mice versus corresponding WT, as well as in KO versus WT three days after TAC, and STAT3 phosphorylation (Y705) in KO versus WT after TAC. *, P<0.01 versus corresponding sham; #, P<0.01 versus corresponding WT (two-way ANOVA with two factors). F. Summary of the findings.
Similar articles
- Cardiac H11 kinase/Hsp22 stimulates oxidative phosphorylation and modulates mitochondrial reactive oxygen species production: Involvement of a nitric oxide-dependent mechanism.
Laure L, Long R, Lizano P, Zini R, Berdeaux A, Depre C, Morin D. Laure L, et al. Free Radic Biol Med. 2012 Jun 1-15;52(11-12):2168-76. doi: 10.1016/j.freeradbiomed.2012.03.001. Epub 2012 Apr 18. Free Radic Biol Med. 2012. PMID: 22542467 - Heat shock protein 22 (Hsp22) regulates oxidative phosphorylation upon its mitochondrial translocation with the inducible nitric oxide synthase in mammalian heart.
Rashed E, Lizano P, Dai H, Thomas A, Suzuki CK, Depre C, Qiu H. Rashed E, et al. PLoS One. 2015 Mar 6;10(3):e0119537. doi: 10.1371/journal.pone.0119537. eCollection 2015. PLoS One. 2015. PMID: 25746286 Free PMC article. - Proteasome activation during cardiac hypertrophy by the chaperone H11 Kinase/Hsp22.
Hedhli N, Wang L, Wang Q, Rashed E, Tian Y, Sui X, Madura K, Depre C. Hedhli N, et al. Cardiovasc Res. 2008 Feb 1;77(3):497-505. doi: 10.1093/cvr/cvm054. Epub 2007 Oct 30. Cardiovasc Res. 2008. PMID: 18006445 - Heat Shock Protein 22 in Physiological and Pathological Hearts: Small Molecule, Large Potentials.
Sun X, Siri S, Hurst A, Qiu H. Sun X, et al. Cells. 2021 Dec 30;11(1):114. doi: 10.3390/cells11010114. Cells. 2021. PMID: 35011676 Free PMC article. Review. - Insights of heat shock protein 22 in the cardiac protection against ischemic oxidative stress.
Wu W, Lai L, Xie M, Qiu H. Wu W, et al. Redox Biol. 2020 Jul;34:101555. doi: 10.1016/j.redox.2020.101555. Epub 2020 Apr 25. Redox Biol. 2020. PMID: 32388268 Free PMC article. Review.
Cited by
- Nucleus, Mitochondrion, or Reticulum? STAT3 à La Carte.
Avalle L, Poli V. Avalle L, et al. Int J Mol Sci. 2018 Sep 18;19(9):2820. doi: 10.3390/ijms19092820. Int J Mol Sci. 2018. PMID: 30231582 Free PMC article. Review. - An Update on the Multifaceted Roles of STAT3 in the Heart.
Harhous Z, Booz GW, Ovize M, Bidaux G, Kurdi M. Harhous Z, et al. Front Cardiovasc Med. 2019 Oct 25;6:150. doi: 10.3389/fcvm.2019.00150. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 31709266 Free PMC article. Review. - Heat Shock Proteins: Protection and Potential Biomarkers for Ischemic Injury of Cardiomyocytes After Surgery.
Santos-Junior VA, Lollo PCB, Cantero MA, Moura CS, Amaya-Farfan J, Morato PN. Santos-Junior VA, et al. Braz J Cardiovasc Surg. 2018 May-Jun;33(3):291-302. doi: 10.21470/1678-9741-2017-0169. Braz J Cardiovasc Surg. 2018. PMID: 30043923 Free PMC article. Review. - Toward a new STATe: the role of STATs in mitochondrial function.
Meier JA, Larner AC. Meier JA, et al. Semin Immunol. 2014 Feb;26(1):20-8. doi: 10.1016/j.smim.2013.12.005. Epub 2014 Jan 14. Semin Immunol. 2014. PMID: 24434063 Free PMC article. Review. - Mitophagy Reduces Oxidative Stress Via Keap1 (Kelch-Like Epichlorohydrin-Associated Protein 1)/Nrf2 (Nuclear Factor-E2-Related Factor 2)/PHB2 (Prohibitin 2) Pathway After Subarachnoid Hemorrhage in Rats.
Zhang T, Wu P, Budbazar E, Zhu Q, Sun C, Mo J, Peng J, Gospodarev V, Tang J, Shi H, Zhang JH. Zhang T, et al. Stroke. 2019 Apr;50(4):978-988. doi: 10.1161/STROKEAHA.118.021590. Stroke. 2019. PMID: 30890112 Free PMC article.
References
- Danan IJ, Rashed ER, Depre C. Therapeutic potential of H11 Kinase for the ischemic heart. Cardiovasc Drug Revs. 2007;25:14–29. - PubMed
- Depre C, Hase M, Gaussin V, Zajac A, Wang L, Hittinger L, Ghaleh B, Yu X, Kudej RK, Wagner T, Sadoshima J, Vatner SF. H11 Kinase is a novel mediator of myocardial hypertrophy in vivo. Circ Res. 2002;91:1007–1014. - PubMed
- Hedhli N, Wang L, Wang Q, Rashed E, Tian Y, Sui X, Madura K, Depre C. Proteasome activation during cardiac hypertrophy by the chaperone H11 Kinase/Hsp22. Cardiovasc Res. 2008;77:497–505. - PubMed
- Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL033107/HL/NHLBI NIH HHS/United States
- P01 AG027211/AG/NIA NIH HHS/United States
- R01 HL033107-27/HL/NHLBI NIH HHS/United States
- R01 HL033107/HL/NHLBI NIH HHS/United States
- HL069020/HL/NHLBI NIH HHS/United States
- R01 HL093415/HL/NHLBI NIH HHS/United States
- R01 HL106511/HL/NHLBI NIH HHS/United States
- R01 HL072863/HL/NHLBI NIH HHS/United States
- A6027211/PHS HHS/United States
- P01 HL069020/HL/NHLBI NIH HHS/United States
- R01 HL093481/HL/NHLBI NIH HHS/United States
- HL 072863/HL/NHLBI NIH HHS/United States
- HL033107/HL/NHLBI NIH HHS/United States
- HL 093415/HL/NHLBI NIH HHS/United States
- R21 HL098802/HL/NHLBI NIH HHS/United States
- P01 HL069020-10/HL/NHLBI NIH HHS/United States
- P01 AG027211-05/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous