Learning-induced changes in mPFC-BLA connections after fear conditioning, extinction, and reinstatement of fear - PubMed (original) (raw)
Comparative Study
. 2011 Oct;36(11):2276-85.
doi: 10.1038/npp.2011.115. Epub 2011 Jul 13.
Affiliations
- PMID: 21750582
- PMCID: PMC3176564
- DOI: 10.1038/npp.2011.115
Comparative Study
Learning-induced changes in mPFC-BLA connections after fear conditioning, extinction, and reinstatement of fear
Rose-Marie Vouimba et al. Neuropsychopharmacology. 2011 Oct.
Abstract
The neural circuit linking the medial prefrontal cortex (mPFC) and the basolateral amygdala (BLA) has crucial roles in both the acquisition and the extinction of fear. However, the mechanism by which this circuit encodes fear and extinction remains unknown. In this study, we monitored changes in the magnitude of evoked field potentials (EFPs) in the mPFC-BLA and BLA-mPFC pathways following auditory fear conditioning and extinction, in freely moving rats. We report that extinction of fear is mediated by depression of the EFPs in the mPFC-BLA and by potentiation in the reciprocal pathway of BLA-mPFC. Interestingly, reinstatement of fear was associated with recovery of freezing and with reversal of the changes in EFPs that were observed following extinction in both pathways. The findings indicate that the mPFC-BLA circuit expresses differential changes following fear and extinction and point to dynamic and plastic changes underlying fear, extinction, and reinstatement. Manipulations targeting these different types of plasticity could constitute a therapeutic tool for the treatment of anxiety disorders.
Figures
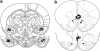
Figure 1
Schematic drawing indicating electrode tip positions within the medial prefrontal cortex (mPFC) and the basolateral amygdala (BLA). Shown is a coronal view of (a) the mPFC and (b) the BLA at position 3.20 mm and 2.70 mm anterior to the bregma, and at −3.14 mm and −3.30 mm posterior to the bregma, respectively. Adapted with permission from Elsevier 1986, Paxinos and Watson, 1986.
Figure 2
Freezing levels following FC and subsequent extinction. The ‘FC only' and ‘FC+Extinction' groups were trained to associate between tone and foot-shock on the FC day. At 24 h following conditioning, the ‘FC+Extinction' group underwent extinction training for three consecutive days (Ext1, Ext2, and Ext3). The ‘Control' group underwent the same protocol as the ‘FC+Extinction' group but in the absence of foot-shocks. The ‘FC+Extinction' showed high freezing levels (calculated as the percentage of time the rats spent freezing during the 30-s tone) on Ext1 (****p<0.001) and Ext2 (***p<0.005), indicating robust acquisition of the association of tone-shock memory. On Ext3, the freezing levels of the ‘FC+Extinction' group were not significantly different from the levels of the ‘Control' group, and thus indicating the success of extinction training. Inset: a subset of the rats that underwent FC had their long-term fear memory tested by re-exposure to the tone 3 days later. These rats show robust retention of fear memory.
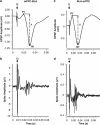
Figure 3
Unit responses and corresponding EFPs. Examples of field potential (a, c) and unit activity (b, d) evoked in the mPFC–BLA (left) and BLA–mPFC (right) pathways. Latency of evoked single-unit activity coincided with peak latency of the negative component of the fields potentials: 24 ms for the mPFC–BLA and 26 ms for BLA–mPFC pathway. St, stimulus artifact. The amplitudes A and A′ of the field potentials (measured from the top peak (P1 or P1′) to the bottom of the sink of the negative wave (N1 or N1′).
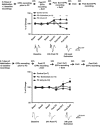
Figure 4
(a) Learning-induced changes in the mPFC–BLA pathway. Top: changes in the EFPs amplitude are expressed as a percentage of change from the baseline recording that was averaged for the two recording days. The EFPs amplitudes 24 h after FC (post FC) were significantly different between the three groups. Post hoc analysis showed a significant increase in EFPs amplitudes for the ‘FC only' and ‘FC+Extinction' groups compared with ‘Control' group, whose EFPs amplitudes did not significantly differ from the baseline levels. By contrast, extinction training was concomitant with a reduction in EFPs amplitudes in the ‘FC+Extinction' group compared with the ‘FC only' and ‘Control' groups post Ext1 and Ext3 (post Ext1, **p<0.005) and post Ext3, ***p<0.001). Bottom: representative traces of the main effects. (b) Learning-induced changes in the BLA–mPFC pathway. Top: FC was not associated with significant changes in EFPs amplitudes 24 h after conditioning. The amplitudes of EFPs were significantly different between the groups on post Ext1 (*p<0.05) and post Ext3 (**p<0.005). Extinction training was accompanied with a significant potentiation of EFPs in the ‘FC+Extinction' group compared with the ‘FC only' and ‘Control groups' (*p<0.005). Bottom: representative traces of the main effects.
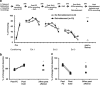
Figure 5
Reinstatement of fear reverses changes following extinction. At 24 h following the last extinction training and after the recording of post Ext3 EFPs, fear was reinstated by placing the animals in the conditioning box in which they either received an unsignaled shock (Reinstatement) or did not receive any shock (No Reinstatement). At 24 h after reinstatement freezing values and EFPs recording were measured. (a) Animals were fear conditioned and for underwent extinction training for 3 days. At 24 h after the last extinction session they were assigned to either No Reinstatement or Reinstatement groups. The results show that the freezing levels of the animals in this experiment (black circles) were not different from those in the previous experiment (dotted line) at any time point. Reinstatement of fear resulted in recovery of the fear responses in the group that received the unsignaled shock compared with the group, which did not undergo reinstatement of fear as 24 h following reinstatement the two groups significantly differ (***p<0.0001). (b) The mPFC–BLA pathway: EFPs were normalized to the values that were reordered during baseline recordings. Similar to the results observed in Figure 4a, FC and extinction of fear were associated with potentiation/depression, respectively. Furthermore, fear was associated with repotentiation of the EFPs compared with baseline values (Reinstatement group: _n_=5, No Reinstatement group, _n_=4; *_p_=0.01). The dotted line represents the data of Figure 3a to show comparable values in the two experiments. (c) The BLA–mPFC pathway: EFPs were normalized to the values that were reordered during baseline recordings. Extinction of fear was associated with potentiation of the EFPs amplitudes and reinstating of fear was associated with depotentiation of the EFPs values as compared with those that were recorded post extinction training (Reinstatement group: _n_=6, No Reinstatement group, _n_=4; *_p_=0.01). The dotted line represents the data of Figure 4b to show comparable values in the two experiments.
Similar articles
- Synaptic encoding of fear extinction in mPFC-amygdala circuits.
Cho JH, Deisseroth K, Bolshakov VY. Cho JH, et al. Neuron. 2013 Dec 18;80(6):1491-507. doi: 10.1016/j.neuron.2013.09.025. Epub 2013 Nov 27. Neuron. 2013. PMID: 24290204 Free PMC article. - Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear.
Laurent V, Westbrook RF. Laurent V, et al. Learn Mem. 2008 Aug 26;15(9):657-66. doi: 10.1101/lm.1080108. Print 2008 Sep. Learn Mem. 2008. PMID: 18772253 - Medial prefrontal cortex: multiple roles in fear and extinction.
Maroun M. Maroun M. Neuroscientist. 2013 Aug;19(4):370-83. doi: 10.1177/1073858412464527. Epub 2012 Oct 22. Neuroscientist. 2013. PMID: 23090707 Review. - Prefrontal mechanisms in extinction of conditioned fear.
Quirk GJ, Garcia R, González-Lima F. Quirk GJ, et al. Biol Psychiatry. 2006 Aug 15;60(4):337-43. doi: 10.1016/j.biopsych.2006.03.010. Epub 2006 May 19. Biol Psychiatry. 2006. PMID: 16712801 Review.
Cited by
- Basolateral Amygdala but Not Medial Prefrontal Cortex Contributes to Chronic Fluoxetine Treatments for PTSD Symptoms in Mice.
Yu YH, Ou CY, Shyu BC, Huang ACW. Yu YH, et al. Behav Neurol. 2020 Nov 25;2020:8875087. doi: 10.1155/2020/8875087. eCollection 2020. Behav Neurol. 2020. PMID: 33299494 Free PMC article. - Paired Electrical Pulse Trains for Controlling Connectivity in Emotion-Related Brain Circuitry.
Lo MC, Younk R, Widge AS. Lo MC, et al. IEEE Trans Neural Syst Rehabil Eng. 2020 Dec;28(12):2721-2730. doi: 10.1109/TNSRE.2020.3030714. Epub 2021 Jan 28. IEEE Trans Neural Syst Rehabil Eng. 2020. PMID: 33048668 Free PMC article. - Enhanced extinction of aversive memories by high-frequency stimulation of the rat infralimbic cortex.
Maroun M, Kavushansky A, Holmes A, Wellman C, Motanis H. Maroun M, et al. PLoS One. 2012;7(5):e35853. doi: 10.1371/journal.pone.0035853. Epub 2012 May 7. PLoS One. 2012. PMID: 22586453 Free PMC article. - Photoperiod alters fear responses and basolateral amygdala neuronal spine density in white-footed mice (Peromyscus leucopus).
Walton JC, Haim A, Spieldenner JM, Nelson RJ. Walton JC, et al. Behav Brain Res. 2012 Aug 1;233(2):345-50. doi: 10.1016/j.bbr.2012.05.033. Epub 2012 May 28. Behav Brain Res. 2012. PMID: 22652395 Free PMC article. - Inactivation of the medial prefrontal cortex interferes with the expression but not the acquisition of differential fear conditioning in rats.
Lee YK, Choi JS. Lee YK, et al. Exp Neurobiol. 2012 Mar;21(1):23-9. doi: 10.5607/en.2012.21.1.23. Epub 2012 Feb 28. Exp Neurobiol. 2012. PMID: 22438676 Free PMC article.
References
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. - PubMed
- Akirav I, Segev A, Motanis H, Maroun M. D-cycloserine into the BLA reverses the impairing effects of exposure to stress on the extinction of contextual fear, but not conditioned taste aversion. Learn Mem. 2009;16:682–686. - PubMed
- Berger TW, Thompson RF. Hippocampal cellular plasticity during extinction of classically conditioned nictitating membrane behavior. Behav Brain Res. 1982;4:63–76. - PubMed
- Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, et al. Role for the Ras signaling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources