Human rhinovirus 2 induces the autophagic pathway and replicates more efficiently in autophagic cells - PubMed (original) (raw)
Human rhinovirus 2 induces the autophagic pathway and replicates more efficiently in autophagic cells
Kathryn A Klein et al. J Virol. 2011 Sep.
Abstract
Picornaviruses rearrange cellular membranes to form cytosolic replication sites. In the case of poliovirus and several other picornaviruses, these membranes are derived from subversion of the cellular autophagy pathway. We also reported observation of autophagosome-like structures during infection by two human rhinoviruses (HRVs), HRV-2 and HRV-14 (W. T. Jackson et al., PLoS Biol. 3:e156, 2005). Another group reported that HRV-2 does not induce autophagosomes or respond to changes in cellular autophagy (M. Brabec-Zaruba, U. Berka, D. Blaas, and R. Fuchs, J. Virol. 81:10815-10817, 2007). In this study, we tested HRV-2-infected cells for activation of autophagic signaling and changes in virus growth in response to changes in autophagy levels. Our data indicate that HRV-2 induces and subverts the autophagic machinery to promote its own replication.
Figures
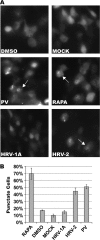
Fig. 1.
Induction of autophagosomes by HRV-2. 293T cells were transfected with a GFP-LC3 expression plasmid. Twenty-four hours later, cells were infected with virus at an MOI of 50, mock infected, or treated with 10 μM rapamycin (RAPA) or DMSO carrier. After 6 h of infection or treatment, cells were fixed for imaging and analysis. (A) Sample images of GFP-LC3 in cells. Arrows indicate a representative cell scored as punctate. (B) Quantification of cells displaying GFP-LC3 puncta. Three random fields, each containing at least 100 cells, were counted. Results from one representative experiment are shown.
Fig. 2.
LC3-II formation in response to HRV-2 infection. 293T cells were infected with PV, HRV-2, or HRV-1A at an MOI of 50. For controls, cells were mock infected or treated with 100 nM bafA for 6 h. At the designated hpi, cell lysates were collected and run on a 13% SDS-PAGE gel, which was probed for LC3 and actin or GAPDH. Unmodified LC3-I and lipid-modified LC3-II are labeled. Quantitation of lipid modification is below each band; intensities were normalized to loading controls, and then the ratio of LC3-II to LC3-I was expressed in arbitrary units.
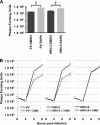
Fig. 3.
HRV-2 replication correlates with autophagic activation. (A) H1-HeLa cells were treated with 10 μM rapamycin for 5 h, then infected with PV or HRV-2 at an MOI of 0.1, and fed with EMEM containing 10 μM rapamycin or an equivalent volume of DMSO carrier. At 6 hpi, cells were collected and cell-associated virus was measured by plaque assay. (B) H1-HeLa cells were infected with PV, HRV-1A, or HRV-2 at an MOI of 0.1 and fed with EMEM containing 10 mM 3-MA. Virus was collected at 0, 2, 4, and 6 h and assayed as described for panel A. Three replicate infections were assayed in each experiment; asterisks indicate significant differences from infections without 3-MA (Student's t test P values of <0.05).
Similar articles
- Interleukin-1 receptor-associated kinase M (IRAK-M) promotes human rhinovirus infection in lung epithelial cells via the autophagic pathway.
Wu Q, van Dyk LF, Jiang D, Dakhama A, Li L, White SR, Gross A, Chu HW. Wu Q, et al. Virology. 2013 Nov;446(1-2):199-206. doi: 10.1016/j.virol.2013.08.005. Epub 2013 Aug 30. Virology. 2013. PMID: 24074582 Free PMC article. - Induction of autophagy does not affect human rhinovirus type 2 production.
Brabec-Zaruba M, Berka U, Blaas D, Fuchs R. Brabec-Zaruba M, et al. J Virol. 2007 Oct;81(19):10815-7. doi: 10.1128/JVI.00143-07. Epub 2007 Aug 1. J Virol. 2007. PMID: 17670838 Free PMC article. - Subversion of cellular autophagosomal machinery by RNA viruses.
Jackson WT, Giddings TH Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K. Jackson WT, et al. PLoS Biol. 2005 May;3(5):e156. doi: 10.1371/journal.pbio.0030156. Epub 2005 Apr 26. PLoS Biol. 2005. PMID: 15884975 Free PMC article. - How positive-strand RNA viruses benefit from autophagosome maturation.
Richards AL, Jackson WT. Richards AL, et al. J Virol. 2013 Sep;87(18):9966-72. doi: 10.1128/JVI.00460-13. Epub 2013 Jun 12. J Virol. 2013. PMID: 23760248 Free PMC article. Review. - Rhinovirus chemotherapy.
Patick AK. Patick AK. Antiviral Res. 2006 Sep;71(2-3):391-6. doi: 10.1016/j.antiviral.2006.03.011. Epub 2006 Apr 18. Antiviral Res. 2006. PMID: 16675037 Free PMC article. Review.
Cited by
- Rhinovirus and Cell Death.
Kerr SL, Mathew C, Ghildyal R. Kerr SL, et al. Viruses. 2021 Apr 7;13(4):629. doi: 10.3390/v13040629. Viruses. 2021. PMID: 33916958 Free PMC article. Review. - Regulation of Cigarette Smoke (CS)-Induced Autophagy by Nrf2.
Zhu L, Barrett EC, Xu Y, Liu Z, Manoharan A, Chen Y. Zhu L, et al. PLoS One. 2013 Apr 9;8(4):e55695. doi: 10.1371/journal.pone.0055695. Print 2013. PLoS One. 2013. PMID: 23585825 Free PMC article. Retracted. - Phosphoinositide-3 kinase inhibition modulates responses to rhinovirus by mechanisms that are predominantly independent of autophagy.
Ismail S, Stokes CA, Prestwich EC, Roberts RL, Juss JK, Sabroe I, Parker LC. Ismail S, et al. PLoS One. 2014 Dec 26;9(12):e116055. doi: 10.1371/journal.pone.0116055. eCollection 2014. PLoS One. 2014. PMID: 25541728 Free PMC article. - A synthetic STING agonist inhibits the replication of human parainfluenza virus 3 and rhinovirus 16 through distinct mechanisms.
Zhu Q, Hu H, Liu H, Shen H, Yan Z, Gao L. Zhu Q, et al. Antiviral Res. 2020 Nov;183:104933. doi: 10.1016/j.antiviral.2020.104933. Epub 2020 Sep 17. Antiviral Res. 2020. PMID: 32949635 Free PMC article. - (+)RNA viruses rewire cellular pathways to build replication organelles.
Belov GA, van Kuppeveld FJ. Belov GA, et al. Curr Opin Virol. 2012 Dec;2(6):740-7. doi: 10.1016/j.coviro.2012.09.006. Epub 2012 Oct 1. Curr Opin Virol. 2012. PMID: 23036609 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases