Adipose tissue remodeling as homeostatic inflammation - PubMed (original) (raw)
Adipose tissue remodeling as homeostatic inflammation
Michiko Itoh et al. Int J Inflam. 2011.
Abstract
Evidence has accumulated indicating that obesity is associated with a state of chronic, low-grade inflammation. Obese adipose tissue is characterized by dynamic changes in cellular composition and function, which may be referred to as "adipose tissue remodeling". Among stromal cells in the adipose tissue, infiltrated macrophages play an important role in adipose tissue inflammation and systemic insulin resistance. We have demonstrated that a paracrine loop involving saturated fatty acids and tumor necrosis factor-α derived from adipocytes and macrophages, respectively, aggravates obesity-induced adipose tissue inflammation. Notably, saturated fatty acids, which are released from hypertrophied adipocytes via the macrophage-induced lipolysis, serve as a naturally occurring ligand for Toll-like receptor 4 complex, thereby activating macrophages. Such a sustained interaction between endogenous ligands derived from parenchymal cells and pathogen sensors expressed in stromal immune cells should lead to chronic inflammatory responses ranging from the basal homeostatic state to diseased tissue remodeling, which may be referred to as "homeostatic inflammation". We, therefore, postulate that adipose tissue remodeling may represent a prototypic example of homeostatic inflammation. Understanding the molecular mechanism underlying homeostatic inflammation may lead to the identification of novel therapeutic strategies to prevent or treat obesity-related complications.
Figures
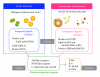
Figure 1
Adipose tissue inflammation as homeostatic inflammation. In innate immunity, exogenous ligands (pathogen-associated molecular patterns; PAMPs) are sensed by pattern-recognition receptors (PRRs), thereby inducing inflammatory changes. On the other hand, damage-associated molecular patterns (DAMPs) released from damaged or stressed cells and tissues can activate PRRs, thereby inducing homeostatic inflammation ranging from the basal homeostatic state to diseased tissue remodeling. For instance, free fatty acids (FFAs) released from hypertrophied adipocytes can report, as a danger signal, their diseased state to macrophages via Toll-like receptor 4 (TLR4) complex during the course of obesity. dsRNA, double-strand RNA; PGN, peptidoglycan; ATP, adenosine tri-phosphate; oxLDL, oxidized low-density lipoprotein; HSP, heat shock protein; HMGB1, high-mobility group box-1.
Figure 2
Molecular mechanism underlying adipose tissue inflammation. During the course of obesity, adipose tissue secretes several chemotactic factors to induce macrophage infiltration into adipose tissue. Circulating monocytes migrate and infiltrate into adipose tissue through adhesion process to endothelial cells. Macrophages enhance the inflammatory changes through the crosstalk with parenchymal adipocytes. For example, the macrophage-derived tumor necrosis factor-α (TNF_α_) induces the release of saturated fatty acids from adipocytes via lipolysis, which, in turn, induces inflammatory changes in macrophages via TLR4. Such a paracrine loop between adipocytes and macrophages constitutes a vicious cycle, thereby further accelerating adipose tissue inflammation. TNF-R, TNF_α_ receptor.
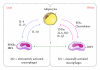
Figure 3
Regulation of macrophage polarity in adipose tissue. Recent evidence has also pointed to the heterogeneity of adipose tissue macrophages, that is, M1 or classically activated (pro-inflammatory) macrophages and M2 or alternatively activated (anti-inflammatory) macrophages. Under lean condition, adipocytes secrete factors that promote M2 activation of macrophages, such as interleukin-4 (IL) and interleukin-13 (IL-13). M2 macrophages secrete anti-inflammatory mediators. On the other hand, adipocytes secrete pro-inflammatory FFAs, chemokines, and cytokines under obese condition. Activated M1 macrophages produce large amounts of pro-inflammatory cytokines, thereby accelerating inflammatory responses in adipose tissue through paracrine interaction between adipocytes and macrophages.
Similar articles
- Adipose tissue macrophages: their role in adipose tissue remodeling.
Suganami T, Ogawa Y. Suganami T, et al. J Leukoc Biol. 2010 Jul;88(1):33-9. doi: 10.1189/jlb.0210072. Epub 2010 Apr 1. J Leukoc Biol. 2010. PMID: 20360405 Review. - Adipose tissue inflammation and ectopic lipid accumulation.
Suganami T, Tanaka M, Ogawa Y. Suganami T, et al. Endocr J. 2012;59(10):849-57. doi: 10.1507/endocrj.ej12-0271. Epub 2012 Aug 9. Endocr J. 2012. PMID: 22878669 Review. - Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated Fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue.
Suganami T, Yuan X, Shimoda Y, Uchio-Yamada K, Nakagawa N, Shirakawa I, Usami T, Tsukahara T, Nakayama K, Miyamoto Y, Yasuda K, Matsuda J, Kamei Y, Kitajima S, Ogawa Y. Suganami T, et al. Circ Res. 2009 Jul 2;105(1):25-32. doi: 10.1161/CIRCRESAHA.109.196261. Epub 2009 May 28. Circ Res. 2009. PMID: 19478204 - Adipocyte-Macrophage Cross-Talk in Obesity.
Engin AB. Engin AB. Adv Exp Med Biol. 2017;960:327-343. doi: 10.1007/978-3-319-48382-5_14. Adv Exp Med Biol. 2017. PMID: 28585206 Review. - Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages.
Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Suganami T, et al. Arterioscler Thromb Vasc Biol. 2007 Jan;27(1):84-91. doi: 10.1161/01.ATV.0000251608.09329.9a. Epub 2006 Nov 2. Arterioscler Thromb Vasc Biol. 2007. PMID: 17082484
Cited by
- Uncovering impaired mitochondrial and lysosomal function in adipose-derived stem cells from obese individuals with altered biological activity.
Wang B, Zhang G, Hu Y, Mohsin A, Chen Z, Hao W, Li Z, Gao WQ, Guo M, Xu H. Wang B, et al. Stem Cell Res Ther. 2024 Jan 8;15(1):12. doi: 10.1186/s13287-023-03625-9. Stem Cell Res Ther. 2024. PMID: 38185703 Free PMC article. - Effects of exercise training on markers of adipose tissue remodeling in patients with coronary artery disease and type 2 diabetes mellitus: sub study of the randomized controlled EXCADI trial.
Zaidi H, Byrkjeland R, Njerve IU, Åkra S, Solheim S, Arnesen H, Seljeflot I, Opstad TB. Zaidi H, et al. Diabetol Metab Syndr. 2019 Dec 19;11:109. doi: 10.1186/s13098-019-0508-9. eCollection 2019. Diabetol Metab Syndr. 2019. PMID: 31890043 Free PMC article. - Aging and adipose tissue: potential interventions for diabetes and regenerative medicine.
Palmer AK, Kirkland JL. Palmer AK, et al. Exp Gerontol. 2016 Dec 15;86:97-105. doi: 10.1016/j.exger.2016.02.013. Epub 2016 Feb 26. Exp Gerontol. 2016. PMID: 26924669 Free PMC article. Review. - Oxidative stress and protein carbonylation in adipose tissue - implications for insulin resistance and diabetes mellitus.
Ruskovska T, Bernlohr DA. Ruskovska T, et al. J Proteomics. 2013 Oct 30;92:323-34. doi: 10.1016/j.jprot.2013.04.002. Epub 2013 Apr 11. J Proteomics. 2013. PMID: 23584148 Free PMC article. Review. - Macrophage Populations in Visceral Adipose Tissue from Pregnant Women: Potential Role of Obesity in Maternal Inflammation.
Bravo-Flores E, Mancilla-Herrera I, Espino Y Sosa S, Ortiz-Ramirez M, Flores-Rueda V, Ibargüengoitia-Ochoa F, Ibañez CA, Zambrano E, Solis-Paredes M, Perichart-Perera O, Sanchez-Martinez M, Medina-Bastidas D, Reyes-Muñoz E, Estrada-Gutierrez G. Bravo-Flores E, et al. Int J Mol Sci. 2018 Apr 4;19(4):1074. doi: 10.3390/ijms19041074. Int J Mol Sci. 2018. PMID: 29617296 Free PMC article.
References
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. - PubMed
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation Research. 2005;96(9):939–949. - PubMed
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nature Reviews Cardiology. 2009;6(6):399–409. - PubMed
- Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines-adipocyte-derived bioactive substances. Annals of the New York Academy of Sciences. 1999;892:146–154. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources