Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders - PubMed (original) (raw)
Review
Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders
Sonsoles Piera-Velazquez et al. Am J Pathol. 2011 Sep.
Abstract
The accumulation of a large number of myofibroblasts is responsible for exaggerated and uncontrolled production of extracellular matrix during the development and progression of pathological fibrosis. Myofibroblasts in fibrotic tissues are derived from at least three sources: expansion and activation of resident tissue fibroblasts, transition of epithelial cells into mesenchymal cells (epithelial-mesenchymal transition, EMT), and tissue migration of bone marrow-derived circulating fibrocytes. Recently, endothelial to mesenchymal transition (EndoMT), a newly recognized type of cellular transdifferentiation, has emerged as another possible source of tissue myofibroblasts. EndoMT is a complex biological process in which endothelial cells lose their specific markers and acquire a mesenchymal or myofibroblastic phenotype and express mesenchymal cell products such as α smooth muscle actin (α-SMA) and type I collagen. Similar to EMT, EndoMT can be induced by transforming growth factor (TGF-β). Recent studies using cell-lineage analysis have demonstrated that EndoMT may be an important mechanism in the pathogenesis of pulmonary, cardiac, and kidney fibrosis, and may represent a novel therapeutic target for fibrotic disorders.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
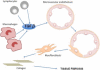
Figure 1
Schematic illustration of the process of EndoMT in tissue fibrosis. TGF-β secreted by tissue-infiltrating chronic inflammatory cells such as macrophages and lymphocytes initiates the transition of endothelial cells into myofibroblasts. These mesenchymal cells of endothelial origin migrate into the interstitium and participate in tissue fibrosis.
Figure 2
Endothelial cell lineage analysis in transgenic mice. The Tie 1 promoter is specific for endothelial cells and drives expression of the Cre recombinase exclusively in endothelial cells and any endothelial lineage cells. The Rosa 26 reporter construct drives expression of a marker (in this illustration, LacZ) and contains two LoxP sites bracketing a stop codon. When Tie 1-Cre mice are crossed with Rosa 26 mice, the Cre recombinase exclusively expressed in endothelial cells and all cells of endothelial lineage will cause excision and deletion of the stop codon bracketed by the LoxP sites in the Rosa26 mice. As a result, all cells from endothelial origin will become labeled with the LacZ marker (blue).
Figure 3
Demonstration of endothelial cell–derived fibroblasts in fibroblast cultures established from lung parenchyma of mice with bleomycin-induced pulmonary fibrosis. Fibroblast cultures were established from lungs from mice injected intratrachealy with either normal saline or bleomycin. When the cultures reached confluency, they were stained with X-Gal to identify the cells from endothelial lineage. Note the absence of X-Gal–staining cells in the cultures from saline-injected control mice (A) in contrast with the marked abundance of X-Gal–staining fibroblasts in the cultures from bleomycin-injected mice (B). The inset in A shows the percentage of X-Gal–positive cells in four separate samples of cultured fibroblasts from saline-injected mice (SLF) compared to eight separate samples of fibroblasts cultured from bleomycin-injected mice (BLF). *P < 0.05. C and D show sequential staining of a fibroblast culture from bleomycin-injected mice with X-Gal (C) followed by immunocytochemistry for the mesenchymal cell markers type I collagen (red) and α- SMA (green). The arrows indicate cells positive for X-Gal, type I collagen, and α-SMA, whereas the arrowheads indicate cells positive for X-Gal and type I collagen.
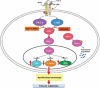
Figure 4
Schematic diagram showing the putative TGF-β signaling pathways involved in EndoMT in pulmonary endothelial cells. Following ligand-initiated activation of the Smad-independent TGF-β pathway, there is phosphorylation of GSK-3β mediated by PKC-δ and the cAbl nonreceptor kinase. Phosphorylation of GSK-3β at serine 9 (ser9) causes its inhibition, which then allows Snail1 to enter the nucleus. Nuclear accumulation of Snail1 results in marked stimulation of Snail1 expression, which then leads to acquisition of the myofibroblast phenotype with stimulation of α-SMA. The inhibition of GSK-3 ser9 phosphorylation by specific inhibition of PKC-δ with rottlerin, or the specific inhibition of c-Abl activity with imatinib, allows GSK-3β to phosphorylate Snail1, targeting it for proteosomal degradation and thus, effectively abolishes the acquisition of the myofibroblastic phenotype and the fibrotic response.
Similar articles
- Myofibroblasts in proliferative diabetic retinopathy can originate from infiltrating fibrocytes and through endothelial-to-mesenchymal transition (EndoMT).
Abu El-Asrar AM, De Hertogh G, van den Eynde K, Alam K, Van Raemdonck K, Opdenakker G, Van Damme J, Geboes K, Struyf S. Abu El-Asrar AM, et al. Exp Eye Res. 2015 Mar;132:179-89. doi: 10.1016/j.exer.2015.01.023. Epub 2015 Jan 28. Exp Eye Res. 2015. PMID: 25637870 - Molecular mechanisms of endothelial to mesenchymal cell transition (EndoMT) in experimentally induced fibrotic diseases.
Piera-Velazquez S, Jimenez SA. Piera-Velazquez S, et al. Fibrogenesis Tissue Repair. 2012 Jun 6;5(Suppl 1):S7. doi: 10.1186/1755-1536-5-S1-S7. eCollection 2012. Fibrogenesis Tissue Repair. 2012. PMID: 23259736 Free PMC article. - Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of Systemic Sclerosis-associated pulmonary fibrosis and pulmonary arterial hypertension. Myth or reality?
Jimenez SA, Piera-Velazquez S. Jimenez SA, et al. Matrix Biol. 2016 Apr;51:26-36. doi: 10.1016/j.matbio.2016.01.012. Epub 2016 Jan 22. Matrix Biol. 2016. PMID: 26807760 Free PMC article. Review. - Endothelial to Mesenchymal Transition (EndoMT) in the Pathogenesis of Human Fibrotic Diseases.
Piera-Velazquez S, Mendoza FA, Jimenez SA. Piera-Velazquez S, et al. J Clin Med. 2016 Apr 11;5(4):45. doi: 10.3390/jcm5040045. J Clin Med. 2016. PMID: 27077889 Free PMC article. Review. - Direct contribution of epithelium to organ fibrosis: epithelial-mesenchymal transition.
Guarino M, Tosoni A, Nebuloni M. Guarino M, et al. Hum Pathol. 2009 Oct;40(10):1365-76. doi: 10.1016/j.humpath.2009.02.020. Epub 2009 Aug 19. Hum Pathol. 2009. PMID: 19695676 Review.
Cited by
- Intercellular communication in peritoneal dialysis.
Sheng L, Shan Y, Dai H, Yu M, Sun J, Huang L, Wang F, Sheng M. Sheng L, et al. Front Physiol. 2024 Feb 8;15:1331976. doi: 10.3389/fphys.2024.1331976. eCollection 2024. Front Physiol. 2024. PMID: 38390449 Free PMC article. Review. - A Decrease in Glomerular Endothelial Cells and Endothelial-mesenchymal Transition during Glomerulosclerosis in the Tensin2-deficient Mice (ICGN strain).
Kato T, Mizuno S, Ito A. Kato T, et al. Acta Histochem Cytochem. 2014;47(6):265-71. doi: 10.1267/ahc.14032. Epub 2014 Nov 21. Acta Histochem Cytochem. 2014. PMID: 25859060 Free PMC article. - Mesenchymal Transitions in Development and Disease.
Medici D, Muñoz-Cánoves P, Yang PC, Brunelli S. Medici D, et al. Stem Cells Int. 2016;2016:5107517. doi: 10.1155/2016/5107517. Epub 2016 Aug 2. Stem Cells Int. 2016. PMID: 27563313 Free PMC article. No abstract available. - Corneal myofibroblasts and fibrosis.
Wilson SE. Wilson SE. Exp Eye Res. 2020 Dec;201:108272. doi: 10.1016/j.exer.2020.108272. Epub 2020 Sep 30. Exp Eye Res. 2020. PMID: 33010289 Free PMC article. Review. - Snail Is a Direct Target of Hypoxia-inducible Factor 1α (HIF1α) in Hypoxia-induced Endothelial to Mesenchymal Transition of Human Coronary Endothelial Cells.
Xu X, Tan X, Tampe B, Sanchez E, Zeisberg M, Zeisberg EM. Xu X, et al. J Biol Chem. 2015 Jul 3;290(27):16653-64. doi: 10.1074/jbc.M115.636944. Epub 2015 May 13. J Biol Chem. 2015. PMID: 25971970 Free PMC article.
References
- Denton C.P., Black C.M. Scleroderma: clinical and pathological advances. Best Pract Res Clin Rheumatol. 2004;18:271–290. - PubMed
- White J.M., Creamer D., du Vivier A.W., Pagliuca A., Ho A.Y., Devereux S., Salisbury J.R., Mufti G.J. Scleroderma graft-versus-host disease: clinical spectrum and therapeutic challenges. Br J Dermatol. 2007;156:1032–1038. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases