Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling - PubMed (original) (raw)
Review
Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling
Arun K Shukla et al. Trends Biochem Sci. 2011 Sep.
Abstract
β-Arrestins, originally discovered to desensitize activated seven transmembrane receptors (7TMRs; also known as G-protein-coupled receptors, GPCRs), are now well established mediators of receptor endocytosis, ubiquitylation and G protein-independent signaling. Recent global analyses of β-arrestin interactions and β-arrestin-dependent phosphorylation events have uncovered several previously unanticipated roles of β-arrestins in a range of cellular signaling events. These findings strongly suggest that the functional roles of β-arrestins are much broader than currently understood. Biophysical studies aimed at understanding multiple active conformations of the 7TMRs and the β-arrestins have begun to unravel the mechanistic basis for the diverse functional capabilities of β-arrestins in cellular signaling.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
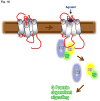
Figure 1
Multifaceted roles of β-arrestins and GRKs in 7TMR signaling and regulation. (a) The classical paradigm of G protein-dependent signaling via 7TMRs where binding of an agonist leads to conformational changes in the receptor. Activated receptor in turn couples to and activates heterotrimeric G proteins. Upon activation, heterotrimeric G proteins dissociate and activated Gα and Gβγ lead to generation of downstream signaling. (b) GRK-mediated phosphorylation and β-arrestin-mediated desensitization of 7TMRs. Agonist occupied activated receptors are phosphorylated by GRKs at serine/threonines primarily in the C-terminus but also in the intracellular loops. Phosphorylated receptors recruit multifunctional adaptor proteins β-arrestins which sterically hinder further G protein coupling to the receptor and in turn lead to receptor desensitization. (c) Novel roles of β-arrestins as endocytosis adaptors, E3 ubiquitin ligase adaptors and the new paradigm of β-arrestin dependent signaling downstream of 7TMRs. Receptor bound β-arrestins also recruit several components of clathrin dependent internalization machinery to the activated receptors and subsequently promote receptor endocytosis via clathrin coated pits. Moreover, β-arrestins also act as adaptors for a number of different E3 ubiquitin ligases to facilitate receptor ubiquitination. Surprisingly, β-arrestins are also capable of scaffolding a number of signaling molecules such as c-Src, Akt and ERK in order to initiate G protein-independent signaling downstream of activated 7TMRs. Please note that the different binding partners of β-arrestins shown in the figure probably do not bind β-arrestins simultaneously.
Figure 1
Multifaceted roles of β-arrestins and GRKs in 7TMR signaling and regulation. (a) The classical paradigm of G protein-dependent signaling via 7TMRs where binding of an agonist leads to conformational changes in the receptor. Activated receptor in turn couples to and activates heterotrimeric G proteins. Upon activation, heterotrimeric G proteins dissociate and activated Gα and Gβγ lead to generation of downstream signaling. (b) GRK-mediated phosphorylation and β-arrestin-mediated desensitization of 7TMRs. Agonist occupied activated receptors are phosphorylated by GRKs at serine/threonines primarily in the C-terminus but also in the intracellular loops. Phosphorylated receptors recruit multifunctional adaptor proteins β-arrestins which sterically hinder further G protein coupling to the receptor and in turn lead to receptor desensitization. (c) Novel roles of β-arrestins as endocytosis adaptors, E3 ubiquitin ligase adaptors and the new paradigm of β-arrestin dependent signaling downstream of 7TMRs. Receptor bound β-arrestins also recruit several components of clathrin dependent internalization machinery to the activated receptors and subsequently promote receptor endocytosis via clathrin coated pits. Moreover, β-arrestins also act as adaptors for a number of different E3 ubiquitin ligases to facilitate receptor ubiquitination. Surprisingly, β-arrestins are also capable of scaffolding a number of signaling molecules such as c-Src, Akt and ERK in order to initiate G protein-independent signaling downstream of activated 7TMRs. Please note that the different binding partners of β-arrestins shown in the figure probably do not bind β-arrestins simultaneously.
Figure 1
Multifaceted roles of β-arrestins and GRKs in 7TMR signaling and regulation. (a) The classical paradigm of G protein-dependent signaling via 7TMRs where binding of an agonist leads to conformational changes in the receptor. Activated receptor in turn couples to and activates heterotrimeric G proteins. Upon activation, heterotrimeric G proteins dissociate and activated Gα and Gβγ lead to generation of downstream signaling. (b) GRK-mediated phosphorylation and β-arrestin-mediated desensitization of 7TMRs. Agonist occupied activated receptors are phosphorylated by GRKs at serine/threonines primarily in the C-terminus but also in the intracellular loops. Phosphorylated receptors recruit multifunctional adaptor proteins β-arrestins which sterically hinder further G protein coupling to the receptor and in turn lead to receptor desensitization. (c) Novel roles of β-arrestins as endocytosis adaptors, E3 ubiquitin ligase adaptors and the new paradigm of β-arrestin dependent signaling downstream of 7TMRs. Receptor bound β-arrestins also recruit several components of clathrin dependent internalization machinery to the activated receptors and subsequently promote receptor endocytosis via clathrin coated pits. Moreover, β-arrestins also act as adaptors for a number of different E3 ubiquitin ligases to facilitate receptor ubiquitination. Surprisingly, β-arrestins are also capable of scaffolding a number of signaling molecules such as c-Src, Akt and ERK in order to initiate G protein-independent signaling downstream of activated 7TMRs. Please note that the different binding partners of β-arrestins shown in the figure probably do not bind β-arrestins simultaneously.
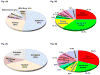
Figure 2
The global scope of the β-arrestin interactome and β-arrestin dependent phosphorylation events. (a) Subcellular and (b) functional distribution of β-arrestin interacting proteins with and without stimulation of the AT1aR by angiotensin II [68]. Note that the majority of β-arrestin interaction partners are distributed in the cytoplasm but a significant fraction are nuclear proteins highlighting the potential nuclear roles of β-arrestins. The functional distribution of β-arrestin binding proteins highlights their major roles in cellular signaling, cellular organization and nucleic acid binding. (c) Subcellular and (d) Functional distribution of the proteins which are phosphorylated upon activation of the AT1aR by a β-arrestin-biased ligand, SII-angiotensin [68]. Note that both the cellular and functional distribution of proteins which are phosphorylated/dephosphorylated upon SII stimulation in a β-arrestin dependent manner mimics the pattern of proteins identified in the interactomics screen.
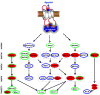
Figure 3
An example of a β-arrestin dependent MAP kinase signaling network downstream of AT1aR. The proteins highlighted in red interact with β-arrestins, the proteins outlined in green are phosphorylated upon activation of AT1aR by a β-arrestin biased ligand, SII-angiotensin. The protein highlighted in red and outlined in green were present in both, the β-arrestin interactome and the β-arrestin phosphoproteome. The proteins outlined in blue are present in the corresponding MAP kinase pathways but are not identified in the proteomics screen. Please note that this figure highlights a potential β-arrestin dependent MAP kinase signaling network based on interactomics and phosphoproteomics studies and all the MAP kinase modules depicted here have not necessarily been documented to be regulated by β-arrestins. The dotted arrows indicate the pathways which have not yet been confirmed by independent studies to be directly activated by β-arrestins, the solid arrows indicate the pathways which have been studied in detail and which are well established to be modulated by β-arrestins.
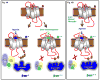
Figure 4
A schematic representation of a simple conceptual framework to explain the mechanistic basis of β-arrestin biased signaling. (a) Binding of an unbiased ligand to the receptor induces an active conformation of the receptor (R*) whereas binding of a β-arrestin-biased ligand promotes a different active conformation of the receptor (R**). The distinct receptor conformations are coupled to corresponding active conformations of β-arrestin (β-arr * and β-arr **) which govern different functional outcomes. (b) Binding of an unbiased agonist to a β-arrestin biased receptor also induces a distinct conformation in the receptor which is likely to be similar to that induced by a β-arrestin-biased ligand to the wild-type receptor and a corresponding conformation in β-arrestin. The exact structural details of these multiple active conformations of receptors and β-arrestins remain to be deciphered by crystallography and other biophysical methods.
Similar articles
- Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR).
Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villén J, Haas W, Kovacs JJ, Shukla AK, Hara MR, Hernandez M, Lachmann A, Zhao S, Lin Y, Cheng Y, Mizuno K, Ma'ayan A, Gygi SP, Lefkowitz RJ. Xiao K, et al. Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):15299-304. doi: 10.1073/pnas.1008461107. Epub 2010 Aug 4. Proc Natl Acad Sci U S A. 2010. PMID: 20686112 Free PMC article. - β-Arrestin-mediated receptor trafficking and signal transduction.
Shenoy SK, Lefkowitz RJ. Shenoy SK, et al. Trends Pharmacol Sci. 2011 Sep;32(9):521-33. doi: 10.1016/j.tips.2011.05.002. Epub 2011 Jun 15. Trends Pharmacol Sci. 2011. PMID: 21680031 Free PMC article. Review. - Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes.
Shenoy SK, Lefkowitz RJ. Shenoy SK, et al. J Biol Chem. 2005 Apr 15;280(15):15315-24. doi: 10.1074/jbc.M412418200. Epub 2005 Feb 7. J Biol Chem. 2005. PMID: 15699045 - Arrestins and protein ubiquitination.
Kommaddi RP, Shenoy SK. Kommaddi RP, et al. Prog Mol Biol Transl Sci. 2013;118:175-204. doi: 10.1016/B978-0-12-394440-5.00007-3. Prog Mol Biol Transl Sci. 2013. PMID: 23764054 Review. - Distinct conformational changes in beta-arrestin report biased agonism at seven-transmembrane receptors.
Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. Shukla AK, et al. Proc Natl Acad Sci U S A. 2008 Jul 22;105(29):9988-93. doi: 10.1073/pnas.0804246105. Epub 2008 Jul 11. Proc Natl Acad Sci U S A. 2008. PMID: 18621717 Free PMC article.
Cited by
- Discovery of positive allosteric modulators and silent allosteric modulators of the μ-opioid receptor.
Burford NT, Clark MJ, Wehrman TS, Gerritz SW, Banks M, O'Connell J, Traynor JR, Alt A. Burford NT, et al. Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10830-5. doi: 10.1073/pnas.1300393110. Epub 2013 Jun 10. Proc Natl Acad Sci U S A. 2013. PMID: 23754417 Free PMC article. - How Carvedilol activates β2-adrenoceptors.
Benkel T, Zimmermann M, Zeiner J, Bravo S, Merten N, Lim VJY, Matthees ESF, Drube J, Miess-Tanneberg E, Malan D, Szpakowska M, Monteleone S, Grimes J, Koszegi Z, Lanoiselée Y, O'Brien S, Pavlaki N, Dobberstein N, Inoue A, Nikolaev V, Calebiro D, Chevigné A, Sasse P, Schulz S, Hoffmann C, Kolb P, Waldhoer M, Simon K, Gomeza J, Kostenis E. Benkel T, et al. Nat Commun. 2022 Nov 19;13(1):7109. doi: 10.1038/s41467-022-34765-w. Nat Commun. 2022. PMID: 36402762 Free PMC article. - Phosphorylation on Ser-279 and Ser-282 of connexin43 regulates endocytosis and gap junction assembly in pancreatic cancer cells.
Johnson KE, Mitra S, Katoch P, Kelsey LS, Johnson KR, Mehta PP. Johnson KE, et al. Mol Biol Cell. 2013 Mar;24(6):715-33. doi: 10.1091/mbc.E12-07-0537. Epub 2013 Jan 30. Mol Biol Cell. 2013. PMID: 23363606 Free PMC article. - The arrestin-domain containing protein AdcA is a response element to stress.
Habourdin C, Klein G, Araki T, Williams JG, Aubry L. Habourdin C, et al. Cell Commun Signal. 2013 Nov 22;11:91. doi: 10.1186/1478-811X-11-91. Cell Commun Signal. 2013. PMID: 24267687 Free PMC article. - Structural evidence for visual arrestin priming via complexation of phosphoinositols.
Sander CL, Luu J, Kim K, Furkert D, Jang K, Reichenwallner J, Kang M, Lee HJ, Eger BT, Choe HW, Fiedler D, Ernst OP, Kim YJ, Palczewski K, Kiser PD. Sander CL, et al. Structure. 2022 Feb 3;30(2):263-277.e5. doi: 10.1016/j.str.2021.10.002. Epub 2021 Oct 21. Structure. 2022. PMID: 34678158 Free PMC article.
References
- Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, et al. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88:263–273. - PubMed
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. - PubMed
- Ma P, Zemmel R. Value of novelty? Nat Rev Drug Discov. 2002;1:571–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL016037-40/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- HL16037/HL/NHLBI NIH HHS/United States
- R01 HL016037/HL/NHLBI NIH HHS/United States
- R01 HL070631/HL/NHLBI NIH HHS/United States
- HL70631/HL/NHLBI NIH HHS/United States
- R01 HL070631-09/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources