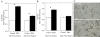Neuroinflammation induces glial aromatase expression in the uninjured songbird brain - PubMed (original) (raw)
Neuroinflammation induces glial aromatase expression in the uninjured songbird brain
Kelli A Duncan et al. J Neuroinflammation. 2011.
Abstract
Background: Estrogens from peripheral sources as well as central aromatization are neuroprotective in the vertebrate brain. Under normal conditions, aromatase is only expressed in neurons, however following anoxic/ischemic or mechanical brain injury; aromatase is also found in astroglia. This increased glial aromatization and the consequent estrogen synthesis is neuroprotective and may promote neuronal survival and repair. While the effects of estradiol on neuroprotection are well studied, what induces glial aromatase expression remains unknown.
Methods: Adult male zebra finches (Taeniopygia guttata) were given a penetrating injury to the entopallium. At several timepoints later, expression of aromatase, IL-1β-like, and IL-6-like were examined using immunohistochemistry. A second set of zebra birds were exposed to phytohemagglutinin (PHA), an inflammatory agent, directly on the dorsal surface of the telencephalon without creating a penetrating injury. Expression of aromatase, IL-1β-like, and IL-6-like were examined using both quantitative real-time polymerase chain reaction to examine mRNA expression and immunohistochemistry to determine cellular expression. Statistical significance was determined using t-test or one-way analysis of variance followed by the Tukey Kramers post hoc test.
Results: Following injury in the zebra finch brain, cytokine expression occurs prior to aromatase expression. This temporal pattern suggests that cytokines may induce aromatase expression in the damaged zebra finch brain. Furthermore, evoking a neuroinflammatory response characterized by an increase in cytokine expression in the uninjured brain is sufficient to induce glial aromatase expression.
Conclusions: These studies are among the first to examine a neuroinflammatory response in the songbird brain following mechanical brain injury and to describe a novel neuroimmune signal to initiate aromatase expression in glia.
Figures
Figure 1
Interleukin antibody specificity and cellular characterization. Western blot analysis of IL-1β-like (A) and IL-6-like (B). Representative high power magnification of IL-1β-like (C) and IL-6-like (G) immunoreactive cells in the zebra finch brain. IL-1β-like (D-F) and IL-6-like (H-J) cells around injury coexpress microglial proteins (yellow, (F, J). Panels (D-E) and (H-I) reveal the identical field of cells viewed through the green (microglia) and red (IL-1β-like and IL-6-like) channels alone.
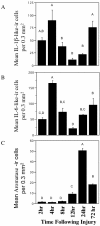
Figure 2
Temporal expression of IL-1β-like immunoreactive cells (A), IL-6-like (B), and Aromatase (C) following mechanical injury in the zebra finch brain. Data are represented as mean ± SEM. Data not connected by the same letter are significantly different (p < .05).
Figure 3
Bar graph depicting the mean uncalibrated optical density (OD) for IL-1β-like (A) and IL-6-like (B) immunoreactive cells at 6 h and 24 h following treatment with PHA. (C) Representative sections depicting the localization of IL-1-like (top) and IL-6-like -ir cells (bottom) following PHA treatment. * denotes a significant difference between treatment and saline.
Figure 4
Bar graph showing the mean ± SEM in ΔCt values (normalized against GAPDH, (A)) and fold change (B) between saline and PHA treated telencephalons for aromatase. Fold change in expression was calculated using the double delta Ct method assuming 100% efficiency. * denotes a significant difference between treatment and saline.
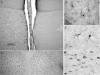
Figure 5
Representative sections from male zebra finches for aromatase immunoreactivity (Arom-ir) following exposure to PHA and saline. (A) Low power magnification of Arom-ir following exposure to PHA (left) and saline (right). (B) Higher power magnification of Arom-ir cells after exposure to PHA. (C) High power magnification exhibiting the typical cellular morphology and structure of Arom-ir cells following exposure to PHA. (D) Control section showing that normal Arom-ir is not affected by treatment with PHA (left) or saline (right). (E) High power magnification showing neuronal expression of aromatase.
Figure 6
Panels (A) and (B) reveal the identical field of cells viewed through the red (aromatase (B)) and green (vimentin (C)) channels alone. Aromatase cells coexpress glial proteins (yellow, C).
Figure 7
Representative sections black-white inverted following cell degeneration assay (TUNEL). TUNEL- labeled cells were not present in tissue exposed to PHA (A). PHA tissue was run concurrently with injured tissue to serve as a positive control (B).
Figure 8
Proposed model of aromatase mediated neuroprotection following injury or damage to the brain.
Similar articles
- Centrally Synthesized Estradiol Is a Potent Anti-Inflammatory in the Injured Zebra Finch Brain.
Pedersen AL, Nelson LH, Saldanha CJ. Pedersen AL, et al. Endocrinology. 2016 May;157(5):2041-51. doi: 10.1210/en.2015-1991. Epub 2016 Mar 10. Endocrinology. 2016. PMID: 26963472 Free PMC article. - Glial aromatization increases the expression of bone morphogenetic protein-2 in the injured zebra finch brain.
Walters BJ, Saldanha CJ. Walters BJ, et al. J Neurochem. 2008 Jul;106(1):216-23. doi: 10.1111/j.1471-4159.2008.05352.x. Epub 2008 Jul 1. J Neurochem. 2008. PMID: 18363824 - Radial glia express aromatase in the injured zebra finch brain.
Peterson RS, Lee DW, Fernando G, Schlinger BA. Peterson RS, et al. J Comp Neurol. 2004 Jul 19;475(2):261-9. doi: 10.1002/cne.20157. J Comp Neurol. 2004. PMID: 15211466 - Traumatized and inflamed--but resilient: glial aromatization and the avian brain.
Duncan KA, Walters BJ, Saldanha CJ. Duncan KA, et al. Horm Behav. 2013 Feb;63(2):208-15. doi: 10.1016/j.yhbeh.2012.02.026. Epub 2012 Mar 5. Horm Behav. 2013. PMID: 22414444 Free PMC article. Review. - Estrogen as a Neuroprotectant in Both Sexes: Stories From the Bird Brain.
Saldanha CJ. Saldanha CJ. Front Neurol. 2020 Jun 23;11:497. doi: 10.3389/fneur.2020.00497. eCollection 2020. Front Neurol. 2020. PMID: 32655477 Free PMC article. Review.
Cited by
- Sex, Genes, and Traumatic Brain Injury (TBI): A Call for a Gender Inclusive Approach to the Study of TBI in the Lab.
Duncan KA, Garijo-Garde S. Duncan KA, et al. Front Neurosci. 2021 May 5;15:681599. doi: 10.3389/fnins.2021.681599. eCollection 2021. Front Neurosci. 2021. PMID: 34025346 Free PMC article. No abstract available. - Central aromatization: A dramatic and responsive defense against threat and trauma to the vertebrate brain.
Duncan KA, Saldanha CJ. Duncan KA, et al. Front Neuroendocrinol. 2020 Jan;56:100816. doi: 10.1016/j.yfrne.2019.100816. Epub 2019 Nov 28. Front Neuroendocrinol. 2020. PMID: 31786088 Free PMC article. Review. - Evaluation of reference genes for quantitative real-time PCR in the brain, pituitary, and gonads of songbirds.
Zinzow-Kramer WM, Horton BM, Maney DL. Zinzow-Kramer WM, et al. Horm Behav. 2014 Jul;66(2):267-75. doi: 10.1016/j.yhbeh.2014.04.011. Epub 2014 Apr 26. Horm Behav. 2014. PMID: 24780145 Free PMC article. - Entopallium Lost GFAP Immunoreactivity during Avian Evolution: Is GFAP a "Condition Sine Qua Non"?
Kálmán M, Sebők OM. Kálmán M, et al. Brain Behav Evol. 2023;98(6):302-313. doi: 10.1159/000535281. Epub 2023 Dec 9. Brain Behav Evol. 2023. PMID: 38071961 Free PMC article. - Differences in vocal brain areas and astrocytes between the house wren and the rufous-tailed hummingbird.
López-Murillo C, Hinestroza-Morales S, Henny P, Toledo J, Cardona-Gómez GP, Rivera-Gutiérrez H, Posada-Duque R. López-Murillo C, et al. Front Neuroanat. 2024 Mar 27;18:1339308. doi: 10.3389/fnana.2024.1339308. eCollection 2024. Front Neuroanat. 2024. PMID: 38601797 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical