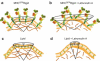Probing orientational behavior of MHC class I protein and lipid probes in cell membranes by fluorescence polarization-resolved imaging - PubMed (original) (raw)
Probing orientational behavior of MHC class I protein and lipid probes in cell membranes by fluorescence polarization-resolved imaging
Alla Kress et al. Biophys J. 2011.
Abstract
Steady-state polarization-resolved fluorescence imaging is used to analyze the molecular orientational order behavior of rigidly labeled major histocompatibility complex class I (MHC I) proteins and lipid probes in cell membranes of living cells. These fluorescent probes report the orientational properties of proteins and their surrounding lipid environment. We present a statistical study of the molecular orientational order, modeled as the width of the angular distribution of the molecules, for the proteins in the cell endomembrane and plasma membrane, as well as for the lipid probes in the plasma membrane. We apply this methodology on cells after treatments affecting the actin and microtubule networks. We find in particular opposite orientational order changes of proteins and lipid probes in the plasma membrane as a response to the cytoskeleton disruption. This suggests that MHC I orientational order is governed by its interaction with the cytoskeleton, whereas the plasma membrane lipid order is governed by the local cell membrane morphology.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
Schematic representation of (a) MHC_GFP_Rigid and (b) MHC_GFP_Flexible. (c) Typical polarimetry responses IX(α) and IY(α) measured for a varying incident polarization angle α, at a given point of a di-8-ANEPPQ-labeled cell membrane. (Continuous lines) Fits (see the Supporting Material). (d) Scheme of a membrane equator representing the molecular orientational cone-shaped distribution with the cone aperture angle ψ of fluorescent probes inserted into the membrane or attached to a membrane protein. The X and Y axes define the sample plane in which the excitation polarization is fixed. The angle ρ specifies the cone orientation in this frame. (e) Anisotropy ratio A(ψ) dependence for ρ = 0° (top line), ρ = 45° (middle line), and ρ = 90° (lower line). This function accounts for polarization distortions in the setup (see text).
Figure 2
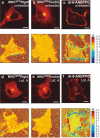
Fluorescence image (top) and corresponding anisotropy images (bottom) of COS-7 cells labeled with (a) MHC_GFP_Rigid, (b) MHC_GFP_Flexible, (c) di-8-ANEPPQ without treatments and (d) MHC_GFP_Rigid, (e) MHC_GFP_Flexible, (f) di-8-ANEPPQ treated with latrunculin A. The typical fluorescence intensity varies between 200 and 500 K counts/s. Scale bar: 10 _μ_m.
Figure 3
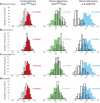
Histograms showing the cone aperture angle distribution ψ before (plain histograms) and after the treatments (line histograms) with (a) hypotonic shock, (b) latrunculin A, (c) cytochalasin D, and (d) colcemid, for MHC_GFP_Rigid in both the endomembrane (left) and the plasma membrane (middle), and di-8-ANEPPQ in the plasma membrane (right). All measurements are performed at 37°C. In each case, the statistics are performed on 200 total areas taken within a population of 5–10 cells.
Figure 4
Proposed scheme for (a) MHC I and (c) di-8-ANEPPQ orientational behavior in the untreated case and (b) MHC I and (d) di-8-ANEPPQ after actin polymerization inhibition with Latrunculin A.
Similar articles
- Mapping the local organization of cell membranes using excitation-polarization-resolved confocal fluorescence microscopy.
Kress A, Wang X, Ranchon H, Savatier J, Rigneault H, Ferrand P, Brasselet S. Kress A, et al. Biophys J. 2013 Jul 2;105(1):127-36. doi: 10.1016/j.bpj.2013.05.043. Biophys J. 2013. PMID: 23823231 Free PMC article. - Intrasequence GFP in class I MHC molecules, a rigid probe for fluorescence anisotropy measurements of the membrane environment.
Rocheleau JV, Edidin M, Piston DW. Rocheleau JV, et al. Biophys J. 2003 Jun;84(6):4078-86. doi: 10.1016/S0006-3495(03)75133-9. Biophys J. 2003. PMID: 12770911 Free PMC article. - Trafficking of MHC molecules to the cell surface creates dynamic protein patches.
Blumenthal D, Edidin M, Gheber LA. Blumenthal D, et al. J Cell Sci. 2016 Sep 1;129(17):3342-50. doi: 10.1242/jcs.187112. Epub 2016 Jul 27. J Cell Sci. 2016. PMID: 27466380 - Fluorescent probes for bacterial cytoplasmic membrane research.
Trevors JT. Trevors JT. J Biochem Biophys Methods. 2003 Aug 29;57(2):87-103. doi: 10.1016/s0165-022x(03)00076-9. J Biochem Biophys Methods. 2003. PMID: 12915003 Review. - Fluorescent small-molecule probes of biochemistry at the plasma membrane.
Cairo CW, Key JA, Sadek CM. Cairo CW, et al. Curr Opin Chem Biol. 2010 Feb;14(1):57-63. doi: 10.1016/j.cbpa.2009.09.032. Epub 2009 Nov 4. Curr Opin Chem Biol. 2010. PMID: 19896411 Review.
Cited by
- Mapping the local organization of cell membranes using excitation-polarization-resolved confocal fluorescence microscopy.
Kress A, Wang X, Ranchon H, Savatier J, Rigneault H, Ferrand P, Brasselet S. Kress A, et al. Biophys J. 2013 Jul 2;105(1):127-36. doi: 10.1016/j.bpj.2013.05.043. Biophys J. 2013. PMID: 23823231 Free PMC article. - Modification of plasma membrane organization in tobacco cells elicited by cryptogein.
Gerbeau-Pissot P, Der C, Thomas D, Anca IA, Grosjean K, Roche Y, Perrier-Cornet JM, Mongrand S, Simon-Plas F. Gerbeau-Pissot P, et al. Plant Physiol. 2014 Jan;164(1):273-86. doi: 10.1104/pp.113.225755. Epub 2013 Nov 14. Plant Physiol. 2014. PMID: 24235133 Free PMC article. - Quantitative nanoscale imaging of orientational order in biological filaments by polarized superresolution microscopy.
Valades Cruz CA, Shaban HA, Kress A, Bertaux N, Monneret S, Mavrakis M, Savatier J, Brasselet S. Valades Cruz CA, et al. Proc Natl Acad Sci U S A. 2016 Feb 16;113(7):E820-8. doi: 10.1073/pnas.1516811113. Epub 2016 Feb 1. Proc Natl Acad Sci U S A. 2016. PMID: 26831082 Free PMC article. - The role of molecular dipole orientation in single-molecule fluorescence microscopy and implications for super-resolution imaging.
Backlund MP, Lew MD, Backer AS, Sahl SJ, Moerner WE. Backlund MP, et al. Chemphyschem. 2014 Mar 17;15(4):587-99. doi: 10.1002/cphc.201300880. Epub 2013 Dec 30. Chemphyschem. 2014. PMID: 24382708 Free PMC article. Review. - Protein exchange is reduced in calcium-independent epithelial junctions.
Bartle EI, Rao TC, Beggs RR, Dean WF, Urner TM, Kowalczyk AP, Mattheyses AL. Bartle EI, et al. J Cell Biol. 2020 Jun 1;219(6):e201906153. doi: 10.1083/jcb.201906153. J Cell Biol. 2020. PMID: 32399559 Free PMC article.
References
- Pentcheva T., Edidin M. Clustering of peptide-loaded MHC class I molecules for endoplasmic reticulum export imaged by fluorescence resonance energy transfer. J. Immunol. 2001;166:6625–6632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials