Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge - PubMed (original) (raw)
Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge
Alexei V Tumanov et al. Cell Host Microbe. 2011.
Abstract
Innate lymphoid cells (ILCs) have emerged as important players, regulating the balance between protective immunity and immunopathology at mucosal surfaces. However, mechanisms that regulate ILCs' effector functions during mucosal pathogenic challenge are poorly defined. Using mice infected with the natural mouse enteric pathogen Citrobacter rodentium, we demonstrate that lymphotoxin (LT) is essential for IL-22 production by intestinal ILCs. Blocking of LTβR signaling dramatically reduced intestinal IL-22 production after C. rodentium infection. Conversely, stimulating LTβR signaling induced an IL-22 protection pathway in LT-deficient mice. Furthermore, exogenous IL-22 expression rescued LTβR-deficient mice. IL-22-producing ILCs were predominantly located in lymphoid follicles in the colon and interacted closely with dendritic cells (DCs). We find that an LT-driven positive feedback loop controls IL-22 production by RORγt(+) ILCs via LTβR signaling in DCs. Taken together, our data show that LTβR signaling in gut lymphoid follicles regulates IL-22 production by ILCs in response to mucosal pathogen challenge.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures
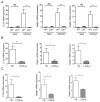
Figure 1. LTβR signaling controls innate IL-22 pathway in the gut
A. IL-22 levels in the colon of naïve and infected WT and _Ltbr_-/- mice. _LTbr_-/- and WT mice (n=5/group/experiment) were orally inoculated with C. rodentium. mRNA expression of IL-22 and IL-22- dependent RegIIIγ and RegIIIβ antimicrobial proteins in the colon were measured by real-time PCR on day 5 post infection. Naïve WT mice were used as control. B. WT mice were treated with 150 μg of LTβR-Ig, or control human Ig (hIg) at day 0 and 3 post infection. Expression of IL-22 and RegIIIγ and RegIIIβ antimicrobial proteins were measured in colon by real-time PCR at day 5 post infection. C. _Rag1_-/- mice were treated intraperitoneally with 150 μg of LTβR-Ig at day 0 and 3 post infection, and expression of IL-22, and RegIIIγ and RegIIIβ antimicrobial proteins was measured by real-time PCR at day 5 post infection. A-C. Data represent means ± s.e.m. n=5, *p<0.05, **p<0.01. Data represent one of three independent experiments with similar results. Real-time PCR data were normalized to hprt expression.
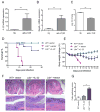
Figure 2. IL-22 is an essential protection pathway downstream of LTβR signaling
A-C. Stimulation of LTβR signaling promotes IL-22 protection pathway. _Ltb_-/- mice were injected intraperitoneally with 75μg of agonistic anti-LTβR antibody or control rat Ig (rIg) at day 0 and 3 post infection with C. rodentium. mRNA expression of IL-22 (A) and RegIIIγ (B) were measured in colon by real-time PCR at day 4 post infection. C. Bacterial titers in liver at day 4 post infection. n=4, **p<0.01, Data represent means ± s.e.m. D-G. Hydrodynamic injection of IL-22 expressing plasmid rescues _Ltbr_-/- mice from lethal C. rodentium infection. IL-22 expressing plasmid or control vector were intravenously injected to WT or _Ltbr_-/-mice 6h after C. rodentium infection. n=10-13/group. Survival (D) and body weight change (E) are shown. ***p<0.001 between IL-22 treated _Ltbr_-/- and control untreated _Ltbr_-/- mice by Mantel-Cox log-rank test. Data combined from two experiments with similar results. F. Representative hematoxylin and eosin staining of colons at day 9 post infection. Red boxes in top panel are shown at higher magnification at lower panel. Bars: 1mm for top panel, and 200μm for lower panel. G. Colitis histopathology score. n=5, *p<0.05. Data represent means ± s.e.m.
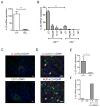
Figure 3. IL-22 is produced in lymphoid follicles by innate RORγt+ cells
A. IL-22 is predominantly expressed in lamina propria. Colon lamina propria (LP) and intraepithelial lymphocyte (IEL) cell populations from WT mice were purified at day 5 post infection. IL-22 expression was measured by real-time PCR. Data normalized to hprt expression. Data represent means ± s.e.m. n=5 mice, **p<0.01. B. LTi cells are predominant IL-22 producing cells in the lamina propria. Colon LP innate cell populations from RORγt-GFP+/-/Ltbr+/+ and RORγt-GFP+/-/_LTbr_-/- mice at day 5 post infection were purified by flow cytometry. IL-22 expression was measured by real-time PCR. Sorted cell populations are indicated as: LTi: CD45+CD3-GFP+NK1.1-NKp46-, NKp46: CD45+CD3-GFP+NKp46+, NK: CD45+CD3-GFP-NK1.1+, Th17: CD45+CD3+GFP+NK1.1-NKp46-. Data combined from two experiments, means ± s.e.m, n=4, **p<0.01. IL-22 data were normalized to hprt expression. C. IL-22 expressing cells are predominantly located in lymphoid follicles. WT mice were orally infected with C. rodentium and colon sections at day 5 post infection were stained with indicated antibodies. Nuclei were stained with DAPI. Bars: 20μm. D. IL-22 producing cells interact with DC in lymphoid follicles. Colon sections of mice at day 5 post infection were stained with indicated antibodies and analyzed by confocal microscopy. Nuclei were stained with DAPI. Arrows indicate contact of DC (green) with IL-22 producing cells (red) on top panel, and RORγt+CD4- cells on bottom panel. Bars: 10μm. E. IL-22 is expressed predominantly in isolated lymphoid follicles. WT mice were orally infected with C. rodentium, and colon tissue collected at day 5 post infection. Isolated lymphoid follicles (ILF+) and surrounded tissue (ILF-) were microdissected under stereo microscope, and IL-22 expression measured by real-time PCR. IL-22 data were normalized to hprt expression. *p<0.05, n=3 mice. F. Co-culture of LTi cells with DC promotes IL-22 production by LTi cells. Lamina propria LTi cells from naïve RORγt-GFP+/- mice were co-cultured in vitro for 18h with lamina propria DCs from WT mice at day 5 post infection. After co-culture, LTi and DC cells were separated by flow cytometry and IL-22 measured by real-time PCR. Data normalized to b-actin. One of two independent experiments with similar results is shown.
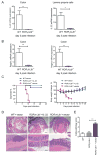
Figure 4. LT expression on RORγt+ cells is essential for control of IL-22 production and protection of mice against C. rodentium infection
A-B. LT expression on RORγt+ cells controls IL-22 protection pathway. WT and RORγt-_Ltb_-/-mice were orally infected with C. rodentium. Expression of IL-22 in colon and purified lamina propria cells (A), and antimicrobial proteins RegIIIγ, and RegIIIβ (B) was measured by real-time PCR at day 5 post infection. Data represent means ± s.e.m. One out of three independent experiments with similar results is shown. n=5 mice, *p<0.05, **p<0.01. Expression data were normalized to hprt expression. C. IL-22 expression is sufficient to rescue RORγt-_Ltb_-/- mice from lethal C. rodentium infection. WT and RORγt-_Ltb_-/- mice were intravenously injected with IL-22 expressing plasmid or control vector at 6h after C. rodentium infection. n=10/group. Data combined from two experiments with similar results. Survival and body weight change are shown. D. Representative hematoxylin and eosin staining of colon at day 9 post infection. Red boxes in top panel are shown at higher magnification at lower panel. Bars: 1mm for top panel, and 200μm for lower panel. E. Colitis histopathology score for mice in panel A. n=5, ***p<0.001. Data represent means ± s.e.m. One of two experiments with similar results is shown.
Figure 5. LTβR signaling in DCs is required for IL-22 production
WT, CD11c-_Ltbr_-/-, and _Ltbr_-/- mice were orally infected with C. rodentium. Body weight change (A) and representative hematoxylin and eosin staining of colon at day 12 post infection (B). Arrows indicate bacterial lesions. Bars: 50μm (top panels), and 20 μm (lower panels). Bacterial titers were measured in feces (C) on day 14, and liver (D), and spleen (E) on day 10 post infection. F. IL-22 expression in colon at day 5 post infection. G. IL-22 levels in colon supernatants at day 5 post infection. H. Expression of RegIIIγ mRNA in colon at day 5 post infection. C-H. Data represent means ± s.e.m. n=5 mice, *p<0.05, **p<0.01. Represents one of three independent experiments with similar results. Real-time PCR data were normalized to hprt expression. I. LTβR signaling in DCs regulates IL-23 production. CD11c+CD11b+ DCs from colon lamina propria of WT and _LTbr_-/- mice were purified by flow cytometry at day 5 post infection. Expression of IL23p19, IL23p40, IL-1b, IL-6 cytokines was measured by real-time PCR. Data combined from two experiments, means ± s.e.m, n=4, *p<0.05, NS-not significant. RT-PCR data normalized to hprt expression.
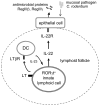
Figure 6. Proposed model to control IL-22 pathway against mucosal bacterial pathogen
LT expression by RORγt+ ILCs is necessary for IL-22 production following invasion of mucosal bacterial pathogen. LT signaling by RORγt+ ILC promotes the development of lymphoid follicles in the gut. Lymphoid follicles provide necessary microenvironment for interaction between innate RORγt+ cells and DC. Interplay between RORγt+ ILCs and DCs promotes IL-23 production by DCs that, in turn, activates IL-22 synthesis by RORγt+ cells as a positive feedback loop. IL-22 stimulates IL-22R on epithelial cells which triggers production of antimicrobial proteins RegIIIγ and RegIIIβ to eliminate mucosal bacterial pathogen.
Comment in
- Another armament in gut immunity: lymphotoxin-mediated crosstalk between innate lymphoid and dendritic cells.
Spits H. Spits H. Cell Host Microbe. 2011 Jul 21;10(1):3-4. doi: 10.1016/j.chom.2011.07.002. Cell Host Microbe. 2011. PMID: 21767806
Similar articles
- Lymphotoxin-β receptor-independent development of intestinal IL-22-producing NKp46+ innate lymphoid cells.
Satoh-Takayama N, Lesjean-Pottier S, Sawa S, Vosshenrich CA, Eberl G, Di Santo JP. Satoh-Takayama N, et al. Eur J Immunol. 2011 Mar;41(3):780-6. doi: 10.1002/eji.201040851. Epub 2011 Feb 1. Eur J Immunol. 2011. PMID: 21341264 - The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells.
Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. Qiu J, et al. Immunity. 2012 Jan 27;36(1):92-104. doi: 10.1016/j.immuni.2011.11.011. Epub 2011 Dec 15. Immunity. 2012. PMID: 22177117 Free PMC article. - Lymphotoxin and the amazing technicolor circus of intestinal homeostasis.
Browning JL. Browning JL. Mucosal Immunol. 2012 May;5(3):228-31. doi: 10.1038/mi.2012.3. Epub 2012 Feb 8. Mucosal Immunol. 2012. PMID: 22318496 - Lymphotoxin organizes contributions to host defense and metabolic illness from innate lymphoid cells.
Upadhyay V, Fu YX. Upadhyay V, et al. Cytokine Growth Factor Rev. 2014 Apr;25(2):227-33. doi: 10.1016/j.cytogfr.2013.12.007. Epub 2013 Dec 24. Cytokine Growth Factor Rev. 2014. PMID: 24411493 Free PMC article. Review. - Heterogeneity and diversity of group 3 innate lymphoid cells: new cells on the block.
Satoh-Takayama N. Satoh-Takayama N. Int Immunol. 2016 Jan;28(1):29-34. doi: 10.1093/intimm/dxv054. Epub 2015 Oct 13. Int Immunol. 2016. PMID: 26462712 Review.
Cited by
- Innate immune response to Salmonella typhimurium, a model enteric pathogen.
Broz P, Ohlson MB, Monack DM. Broz P, et al. Gut Microbes. 2012 Mar-Apr;3(2):62-70. doi: 10.4161/gmic.19141. Epub 2012 Mar 1. Gut Microbes. 2012. PMID: 22198618 Free PMC article. Review. - Innate Lymphoid Cells Control Early Colonization Resistance against Intestinal Pathogens through ID2-Dependent Regulation of the Microbiota.
Guo X, Liang Y, Zhang Y, Lasorella A, Kee BL, Fu YX. Guo X, et al. Immunity. 2015 Apr 21;42(4):731-43. doi: 10.1016/j.immuni.2015.03.012. Immunity. 2015. PMID: 25902484 Free PMC article. - Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium.
Schreiber HA, Loschko J, Karssemeijer RA, Escolano A, Meredith MM, Mucida D, Guermonprez P, Nussenzweig MC. Schreiber HA, et al. J Exp Med. 2013 Sep 23;210(10):2025-39. doi: 10.1084/jem.20130903. Epub 2013 Sep 16. J Exp Med. 2013. PMID: 24043764 Free PMC article. - Sensory Nociceptive Neurons Contribute to Host Protection During Enteric Infection With Citrobacter rodentium.
Ramirez VT, Sladek J, Godinez DR, Rude KM, Chicco P, Murray K, Brust-Mascher I, Gareau MG, Reardon C. Ramirez VT, et al. J Infect Dis. 2020 Jun 11;221(12):1978-1988. doi: 10.1093/infdis/jiaa014. J Infect Dis. 2020. PMID: 31960920 Free PMC article. - Role of G-Protein Coupled Receptors in Chemotaxis of Innate Lymphoid Cells.
Bhatt B, Zhu H, Patel N, Singh N. Bhatt B, et al. Methods Mol Biol. 2020;2121:93-98. doi: 10.1007/978-1-0716-0338-3_9. Methods Mol Biol. 2020. PMID: 32147789 Free PMC article.
References
- Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. - PubMed
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. - PubMed
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. - PubMed
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA134563/CA/NCI NIH HHS/United States
- DK58891/DK/NIDDK NIH HHS/United States
- R01 DK080736/DK/NIDDK NIH HHS/United States
- R21 AI090392/AI/NIAID NIH HHS/United States
- AI062026/AI/NIAID NIH HHS/United States
- CA115540/CA/NCI NIH HHS/United States
- R01 AI062026/AI/NIAID NIH HHS/United States
- R01 CA141975/CA/NCI NIH HHS/United States
- R01 CA115540/CA/NCI NIH HHS/United States
- R01 DK058891/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources