Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration - PubMed (original) (raw)
Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration
Anja Mai et al. J Cell Biol. 2011.
Abstract
Integrin trafficking from and to the plasma membrane controls many aspects of cell behavior including cell motility, invasion, and cytokinesis. Recruitment of integrin cargo to the endocytic machinery is regulated by the small GTPase Rab21, but the detailed molecular mechanisms underlying integrin cargo recruitment are yet unknown. Here we identify an important role for p120RasGAP (RASA1) in the recycling of endocytosed α/β1-integrin heterodimers to the plasma membrane. Silencing of p120RasGAP attenuated integrin recycling and augmented cell motility. Mechanistically, p120RasGAP interacted with the cytoplasmic domain of integrin α-subunits via its GAP domain and competed with Rab21 for binding to endocytosed integrins. This in turn facilitated exit of the integrin from Rab21- and EEA1-positive endosomes to drive recycling. Our results assign an unexpected role for p120RasGAP in the regulation of integrin traffic in cancer cells and reveal a new concept of competitive binding of Rab GTPases and GAP proteins to receptors as a regulatory mechanism in trafficking.
Figures
Figure 1.
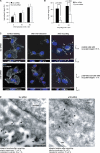
p120RasGAP is required for β1-integrin recycling to the plasma membrane. (A) Serum-starved cells were surface labeled with cleavable biotin. Internalization was allowed for the times indicated. After cleavage of surface remaining biotin, the cells were lysed and immunoprecipitated with anti–β1-integrin antibody, immunoblotted with anti-biotin antibody, followed by reprobing with anti-β1-integrin antibody. The graph shows biotinylated integrin relative to the total amount of surface-biotinylated integrin normalized against immunoprecipitated β1-integrin (mean ± SEM; three independent experiments). (B) Cells were treated as in A, but after internalization (30 min) and removal of cell-surface biotin, recycling (indicated times) was enabled by serum stimulation followed by a second cell-surface biotin cleavage. Recycling was assessed by the decrease of biotinylated integrin (mean ± SEM; six independent experiments). (C) Surface integrins of starved control (Scr) or p120RasGAP-silenced cells (asterisk, based on 3′ Alexa 647–conjugated p120RasGAP-siRNA) were labeled with anti–β1-integrin antibody (gray). Internalization (30 min) was followed by serum-induced recycling for 30 min (arrows; recycled integrins at the plasma membrane). Numbers indicate the percentage of cells with recycled integrins (n = 38–43 cells). Images represent projections of 25–35 planes per stack viewed along the x-y or x-z axis. (D) Recycling assay done as in C, but with 10-nm gold–conjugated anti–β1-integrin antibody and detected with electron microscopy (PM, plasma membrane). Arrows point to gold-labeled integrins. Integrin location after recycling is indicated (n = 35–50 cells scored). Bars: (C)10 µm; (D) 0.2 µm.
Figure 2.
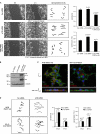
p120RasGAP regulates cell migration downstream of Rab21-driven integrin endocytosis. (A) Migration analysis of control (Scr), p120-silenced MDA-MB-231 cells, or cells that were first silenced for p120RasGAP and then rescued by GFP-p120 expression (p120 siRNA + GFP-p120) by scratch-wound assay. Shown are representative wounds at the indicated times and percentage of wound closure (wound area after [4 h] related to wound area before [0 h] imaging; mean ± SEM, n = 10 wounds per treatment). Cell motility has been analyzed in more detail by tracking the location of cells (n = 50 cells per treatment) over time and measuring both the persistence time (persistence) using the calculated mean squared displacement (MSD) of tracked cells as well as the migration speed (distance migrated per minute). Bar, 100 µm. (B) Western blot analysis with the indicated antibodies from cell lysates of the experiment shown in A. (C) KF28 and KFr13 cells were fixed and immunostained as indicated. Images represent projections covering in total 10–13 µm viewed along the x-y or x-z axis. Bar, 10 µm. (D) Migration of p120RasGAP- or control- (Scr) silenced KF28 and KFr13 cells at the edge of a scratch wound was analyzed using time-lapse microscopy. Shown are representative tracks. Speed and directionality of motility was scored as described in A (mean ± SEM, n = 56–68 cells, combined data from two separate experiments).
Figure 3.
p120RasGAP does not function as a GAP for Rab21. (A) The GTP-hydrolysis–stimulating activity of the recombinantly purified GAP domain of p120RasGAP (p120 GAP) toward Rab21 and H-Ras was measured in a radioactive GAP-assay. 1 µM of Rab21 or H-Ras bound to γ-[32P]GTP was incubated with 5 µM of p120 GAP. The release of the radioactive labeled reaction product 32Pi was detected at the indicated time points by scintillation counting and compared with the total counts per minute used in the assay (cpm ratio). (B) His-APPL1 binding to GST-Rab21–bound beads was studied to confirm successful loading of Rab21 with the GTP analogue GppNHp for the experiment shown in D. (C) Coomassie-stained SDS-PAGE of all recombinant purified proteins used in this study. 7 µg of each protein was loaded to a 10% SDS gel. All proteins are highly pure and degradation could only be observed to some extent for p120. (D) A fluorescence polarization (FP)–based assay was applied to detect binding between 5 µM EDANS-conjugated α2-integrin cytoplasmic domain peptide and different concentrations of GST or GST-Rab21 loaded either with GDP or GppNHp. Complex formation was deduced from an increase in anisotropy signal.
Figure 4.
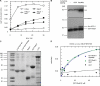
p120RasGAP GAP domain interacts with integrin α-tails. (A) Lysates from MDA-MB-231 cells were subjected to immunoprecipitation with the indicated antibodies. (B) Schematic illustration of the p120RasGAP constructs used. Cell lysate was incubated with immobilized GST or GST-tagged Rab21 and p120RasGAP constructs. Bound proteins were probed for β1-integrin heterodimers. Coomassie-stained SDS-PAGE was used to check for equal loading of the GST fusion proteins. (C) Biotinylated peptides corresponding to the cytoplasmic domain of either α1- or β1-integrin were immobilized and incubated with purified GST-tagged proteins as stated in the figure. Ponceau staining demonstrates usage of equal amounts of the biotinylated peptides. (D) Fluorescence polarization (FP)–based assay to measure the binding between 5 µM EDANS-α2 integrin peptide and different concentrations of the indicated recombinant proteins. Note that in FP-based assays the stronger increase of anisotropy with the full-length p120RasGAP protein is due to higher molecular weight of the protein compared with only the GAP domain of p120. (E) 5 µM of the EDANS-α2-integrin peptide was titrated with increasing concentrations of p120 or p120 GAP to determine equilibrium dissociation constants (Kd).
Figure 5.
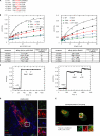
p120RasGAP and Rab21 bind integrin α-tails mutually exclusive. (A) Illustration of the integrin mutant peptides used. (B) Quantitative determination of affinity-binding constants of p120RasGAP and Rab21 with either wild-type (WT) α2-integrin peptide or α2-peptide mutants as stated in the figure. Affinity values in the table are mean values (±SEM) from five independent measurements. The graphs show representative titration curves to determine affinities. (C) FP-based competition assay of Rab21 (25 kD) and p120 (100 kD) for binding to the integrin α2-tail. Left: 5 µM EDANS-α2 peptide, 50 µM p120, and two times 10 µM Rab21 were added consecutively. Right: first, 60 µM Rab21 was added to 5 µM EDANS-α2 peptide. Saturation of the peptide with Rab21 is shown by adding further 10 µM Rab21. Addition of only 10 µM p120 leads then to an increase of anisotropy. (D) Three-color immunostaining of mDsRed-Rab21 (red)–transfected MDA-MB-231 cells with p120RasGAP in green and β1-integrin in blue (note that this is steady-state integrin distribution in cells, not internalized pool of integrin from the cell surface like in Figs. 1 C and 7 A). Bar, 10 µm. (E) To increase colocalization of p120RasGAP and integrins in MDA-MB-231 cells, integrin trafficking was allowed for 45 min in the presence of primaquine (inhibits recycling) after labeling of surface integrins with anti–β1-integrin antibody (red). Cells were then fixed, permeabilized, and stained for endogenous p120RasGAP (green). The determination of the Pearson’s correlation coefficient was done with ImageJ software (n = 27 cells).
Figure 6.
p120 GAP domain, but not p120RasGAP catalytic activity, is crucial for the regulation of cell migration. (A) MDA-MB-231 cells were first silenced with 3′ Alexa 647–conjugated p120RasGAP-siRNA for 2 d followed by transfection with the indicated GFP constructs for 24 h. The cells were then labeled with anti–β1-integrin antibody, and integrin trafficking was allowed for 60 min. In immunostainings of p120RasGAP-siRNA and GFP-positive cells, the amount of integrin recycling to the cell surface was determined by measuring both internal and total integrin staining (n = 15–20 cells each treatment from three independent experiments). (B) Western blot analysis from experiment in C demonstrating silencing and rescue efficiency. (C) P120RasGAP-silenced MDA-MB-231 cells were transfected with the indicated GFP constructs and then allowed to migrate on tissue culture plates for 10 h. Time-lapse images were taken every 10 min for 10 h. Shown are representative track plots. (D) Quantitation of the migration speed of tracked cells from three independent experiments shown in C (mean ± SEM, n = 30 cells per treatment).
Figure 7.
Integrins accumulate in Rab21 endosomes upon p120RasGAP silencing. (A) Surface integrins of starved control (Scr) or p120RasGAP-silenced MDA-MB-231 cells were labeled with anti–β1-integrin antibody (green). Cells were allowed to undergo internalization or recycling as described in Fig. 1 C. Cells were stained for endogenous Rab21 (red) and nuclei (DAPI). Adjacent graphs show the results of line scan analysis of the enlarged areas shown in the figure. Bar, 10 µm. Images were further analyzed for colocalization (Pearson’s correlation coefficient) of Rab21 and β1-integrin in endosomes (mean ± SEM, n = 15 cells). (B) To characterize the compartment in which integrins accumulate upon loss of p120RasGAP, surface integrins in p120-silenced MDA-MB-231 cells were first labeled with anti–β1-integrin antibody (green) and then allowed to undergo recycling. After fixation and permeabilization, the cells were stained for either EEA1, Rab11, or RCP (red) as indicated in the figure. (C) Rab21-EGFP–expressing cells, either control or p120RasGAP silenced, were surface labeled with 10-nm gold–conjugated anti–β1-integrin antibody (black arrows). Subsequently, cells were allowed to undergo internalization followed by serum-induced recycling for 30 min, respectively. Rab21-EGFP was co-labeled with anti-GFP antibody and 5-nm protein A gold (white arrows). Shown are electron microscopic images of cells from frozen thin cryosections (PM, plasma membrane; cyt, cytosol). The quantitation of the ratio of Rab21 gold particles colocalizing with integrins on endosomes (mean ± SEM, n = 40 cells) is indicated. Bar, 200 nm.
Figure 8.
Model of the mechanism of integrin trafficking jointly controlled by Rab21 and p120RasGAP. (A) Rab21 mediates endocytosis of integrins by binding directly to the cytoplasmic tails of the α-integrin subunits. After internalization, p120RasGAP competes for Rab21 binding sites on the integrin α-tail. The replacement of Rab21 by p120RasGAP on early endosomes then triggers the recycling of integrins back to the plasma membrane. (B) Expression of a dominant-negative Rab21 (GDP-locked mutant) impedes integrin internalization and results in diminished cell migration (Pellinen et al., 2006). (C) Loss of p120RasGAP, on the other hand, results in the accumulation of integrins inside the cell in Rab21-positive early endosomes. Yellow bar indicates Rab11.
Similar articles
- Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins.
Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J. Pellinen T, et al. J Cell Biol. 2006 Jun 5;173(5):767-80. doi: 10.1083/jcb.200509019. J Cell Biol. 2006. PMID: 16754960 Free PMC article. - Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane.
Bradley WD, Hernández SE, Settleman J, Koleske AJ. Bradley WD, et al. Mol Biol Cell. 2006 Nov;17(11):4827-36. doi: 10.1091/mbc.e06-02-0132. Epub 2006 Sep 13. Mol Biol Cell. 2006. PMID: 16971514 Free PMC article. - Rab35 GTPase: A Central Regulator of Phosphoinositides and F-actin in Endocytic Recycling and Beyond.
Klinkert K, Echard A. Klinkert K, et al. Traffic. 2016 Oct;17(10):1063-77. doi: 10.1111/tra.12422. Epub 2016 Jul 14. Traffic. 2016. PMID: 27329675 Review. - Rab GTPases-cargo direct interactions: fine modulators of intracellular trafficking.
Aloisi AL, Bucci C. Aloisi AL, et al. Histol Histopathol. 2013 Jul;28(7):839-49. doi: 10.14670/HH-28.839. Epub 2013 Apr 5. Histol Histopathol. 2013. PMID: 23558751 Review.
Cited by
- Conformationally active integrin endocytosis and traffic: why, where, when and how?
Mana G, Valdembri D, Serini G. Mana G, et al. Biochem Soc Trans. 2020 Feb 28;48(1):83-93. doi: 10.1042/BST20190309. Biochem Soc Trans. 2020. PMID: 32065228 Free PMC article. Review. - High-content analysis of Rab protein function at the ER-Golgi interface.
Galea G, Simpson JC. Galea G, et al. Bioarchitecture. 2015;5(3-4):44-53. doi: 10.1080/19490992.2015.1102826. Epub 2015 Dec 22. Bioarchitecture. 2015. PMID: 26693811 Free PMC article. - Protease Substrate-Independent Universal Assay for Monitoring Digestion of Native Unmodified Proteins.
Vuorinen E, Valtonen S, Hassan N, Mahran R, Habib H, Malakoutikhah M, Kopra K, Härmä H. Vuorinen E, et al. Int J Mol Sci. 2021 Jun 14;22(12):6362. doi: 10.3390/ijms22126362. Int J Mol Sci. 2021. PMID: 34198602 Free PMC article. - Integrin traffic - the update.
De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J. De Franceschi N, et al. J Cell Sci. 2015 Mar 1;128(5):839-52. doi: 10.1242/jcs.161653. Epub 2015 Feb 6. J Cell Sci. 2015. PMID: 25663697 Free PMC article. Review. - Exploring the Role of miR-132 in Rat Bladders and Human Urothelial Cells during Wound Healing.
Chamorro CI, Fossum M. Chamorro CI, et al. Int J Mol Sci. 2024 Oct 14;25(20):11039. doi: 10.3390/ijms252011039. Int J Mol Sci. 2024. PMID: 39456820 Free PMC article.
References
- Anand S., Majeti B.K., Acevedo L.M., Murphy E.A., Mukthavaram R., Scheppke L., Huang M., Shields D.J., Lindquist J.N., Lapinski P.E., et al. 2010. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 16:909–914 10.1038/nm.2186 - DOI - PMC - PubMed
- Caswell P.T., Spence H.J., Parsons M., White D.P., Clark K., Cheng K.W., Mills G.B., Humphries M.J., Messent A.J., Anderson K.I., et al. 2007. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell. 13:496–510 10.1016/j.devcel.2007.08.012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous