Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo - PubMed (original) (raw)
Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo
Fredrik H Sterky et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Mitochondrial dysfunction is heavily implicated in Parkinson disease (PD) as exemplified by the finding of an increased frequency of respiratory chain-deficient dopamine (DA) neurons in affected patients. An inherited form of PD is caused by impaired function of Parkin, an E3 ubiquitin ligase reported to translocate to defective mitochondria in vitro to facilitate their clearance. We have developed a reporter mouse to assess mitochondrial morphology in DA neurons in vivo and show here that respiratory chain deficiency leads to fragmentation of the mitochondrial network and to the formation of large cytoplasmic bodies derived from mitochondria. Surprisingly, the dysfunctional mitochondria do not recruit Parkin in vivo, and neither the clearance of defective mitochondria nor the neurodegeneration phenotype is affected by the absence of Parkin. We also show that anterograde axonal transport of mitochondria is impaired in respiratory chain-deficient DA neurons, leading to a decreased supply of mitochondria to the axonal terminals.
Conflict of interest statement
Conflict of interest statement: L.O. and N-G.L. are co-owners of Kampavata AB, which owns commercial rights to the MitoPark mouse.
Figures
Fig. 1.
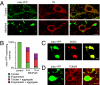
Design of a lox-Stop-lox-mito-YFP cassette integrated into the ROSA26 locus and visualization of mitochondria in DA neurons. (A) Schematic illustration of the lox-Stop-lox-mito-YFP construct. The construct is driven by the endogenous ROSA26 promoter. MTS, mitochondrial targeting presequence lox, heterologous loxP-site lox2372 (
Fig. S1
). (B) Targeting (lox-Stop-lox) and recombination (lox) of the ROSA26 locus as detected by PCR in DNA from SN, cortex (Cx), cerebellum (Cbm), and heart and liver from wild-type ROSA26+/SmY and ROSA26+/SmY;DAT-cre mice. (C) SNc DA neurons in a ROSA26+/SmY;DAT-cre mouse. DA neurons are labeled with an antibody against TH. Quantification showed that 100% (n = 388) of SNc neurons that expressed YFP also were TH immunoreactive and that 99.5% (n = 386) of TH+ cells also expressed mito-YFP. (D) Confocal image of a neuron expressing mito-YFP (green) and labeled with an antibody against the mitochondrial protein TOM20 (red). (Scale bars: 100 μm in C; 5 μm in D.)
Fig. 2.
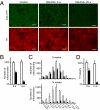
Morphology of mitochondria in normal and respiratory chain-deficient DA neurons. (A) Confocal images of YFP-labeled mitochondria in DA neurons of 8-wk-old control (genotype Tfam+/+;DAT-cre;ROSA26+/SmY) and MitoPark (genotype _Tfam_loxP/loxP;DAT-cre;ROSA26+/SmY) mice. DA neurons also are identified by being TH immunoreactive (red). The detector gain of the YFP signal was decreased in the lower panel to prevent overexposure of the large aggregates. (B) Quantification of mitochondrial morphologies in neurons of control and MitoPark mice at 8 and 14 wk of age (
Fig. S3
). Data are normalized to the total numbers of cells counted separately. (C) MitoPark DA neuron expressing mito-YFP and labeled with an antibody against the mitochondrial matrix protein SOD2 (red). (D) Mitochondrial aggregates immunolabeled with an antibody against the mitochondrial outer membrane protein TOM20 (red). (Scale bar: 10 μm in A; 5 μm in C and D.)
Fig. 3.
Changes in the distal pool of mitochondria. (A) Epifluorescent images of mitochondria and dopaminergic fibers in striatum of control and MitoPark mice at age 8 and 20 wk. (Scale bars: 10 μm.) (B) Number of mitochondria in striatum of control and MitoPark mice at age 8 and 14 wk. (C) Size histograms showing volumes of YFP+ particles counted in B. (D) Denervation at 8 and 14 wk as measured by the area of optical sections occupied with TH-immunoreactive fibers. Open bars, controls; filled bars, MitoPark. Error bars indicate SEM. n = 3.
Fig. 4.
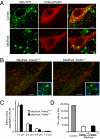
Effect of Parkin on MitoPark pathology. (A) Control and MitoPark DA neurons expressing Cherry-Parkin 3 d after stereotactic viral delivery to the midbrain (age 7 wk). (Scale bars: 5 μm.) (B) Formation of large mitochondria-derived intracellular aggregates in midbrain DA neurons of 8-wk-old MitoPark mice with and without Parkin, respectively. YFP is shown in green, TH in red, and DAPI in blue. (Scale bars: 100 μm.) Insets show enlarged view. (Scale bars: 10 μm.) (C) Average number of enlarged mitochondria in the cell bodies of nigral DA neurons in MitoPark mice with and without Parkin (n = 3). (D) Stereological estimates of the number of TH-immunoreactive cells in bilateral SNc of ∼30-w-old MitoPark mice with and without Parkin (n = 3).
Fig. 5.
Uptake and axonal transport of mitochondrial red fluorescent protein. (A) Confocal images of double-labeled control and MitoPark neurons that express both mito-YFP and mito-dsRed 1 wk after stereotactic injections of AAV-mito-dsRed to the SNc. Mice were analyzed at age 8 wk. (B) Box plots showing dsRed intensities in small mitochondria and large mitochondria-derived aggregates (Aggr) in transduced control and MitoPark neurons (n ≥ 37 cells). Mice were analyzed at age 8 wk. (C) Example of a MitoPark neuron with heterogeneous uptake of mito-dsRed 1 wk after viral delivery. The YFP signal from the enlarged mitochondria was saturated to allow visualization of smaller mitochondria. (D) High-magnification image of striatal areas with mitochondria that contain YFP and dsRed. (E) Quantification of YFP-labeled mitochondria (green bar) and YFP-labeled mitochondria that also contain dsRed (red bar) in striatum of MitoPark mice and controls 1 wk after viral delivery to the SNc. Percentages relate to the number of mitochondria counted in controls (3,137). (F) Number of double-labeled striatal mitochondria normalized by the number of double-labeled SNc neurons (n = 6). (G) Proportion of mito-YFP+, fluorogold–labeled SNc neurons in 8-wk-old mice 48 h after striatal injections of fluorogold (n = 4). (Scale bars: 10 μm in A; 5 μm in C; 2 μm in D.)
Similar articles
- Mitofusin 2 is necessary for striatal axonal projections of midbrain dopamine neurons.
Lee S, Sterky FH, Mourier A, Terzioglu M, Cullheim S, Olson L, Larsson NG. Lee S, et al. Hum Mol Genet. 2012 Nov 15;21(22):4827-35. doi: 10.1093/hmg/dds352. Epub 2012 Aug 21. Hum Mol Genet. 2012. PMID: 22914740 - Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress.
Pickrell AM, Huang CH, Kennedy SR, Ordureau A, Sideris DP, Hoekstra JG, Harper JW, Youle RJ. Pickrell AM, et al. Neuron. 2015 Jul 15;87(2):371-81. doi: 10.1016/j.neuron.2015.06.034. Neuron. 2015. PMID: 26182419 Free PMC article. - Compartmentalized Regulation of Parkin-Mediated Mitochondrial Quality Control in the Drosophila Nervous System In Vivo.
Sung H, Tandarich LC, Nguyen K, Hollenbeck PJ. Sung H, et al. J Neurosci. 2016 Jul 13;36(28):7375-91. doi: 10.1523/JNEUROSCI.0633-16.2016. J Neurosci. 2016. PMID: 27413149 Free PMC article. - PINK1-Parkin signaling in Parkinson's disease: Lessons from Drosophila.
Imai Y. Imai Y. Neurosci Res. 2020 Oct;159:40-46. doi: 10.1016/j.neures.2020.01.016. Epub 2020 Feb 6. Neurosci Res. 2020. PMID: 32035987 Review. - Mitochondrial dysfunction and Parkinson disease: a Parkin-AMPK alliance in neuroprotection.
Hang L, Thundyil J, Lim KL. Hang L, et al. Ann N Y Acad Sci. 2015 Sep;1350:37-47. doi: 10.1111/nyas.12820. Epub 2015 Jun 29. Ann N Y Acad Sci. 2015. PMID: 26121488 Review.
Cited by
- Loss of Mfn2 results in progressive, retrograde degeneration of dopaminergic neurons in the nigrostriatal circuit.
Pham AH, Meng S, Chu QN, Chan DC. Pham AH, et al. Hum Mol Genet. 2012 Nov 15;21(22):4817-26. doi: 10.1093/hmg/dds311. Epub 2012 Jul 31. Hum Mol Genet. 2012. PMID: 22859504 Free PMC article. - Defects in Mitochondrial Biogenesis Drive Mitochondrial Alterations in PARKIN-Deficient Human Dopamine Neurons.
Kumar M, Acevedo-Cintrón J, Jhaldiyal A, Wang H, Andrabi SA, Eacker S, Karuppagounder SS, Brahmachari S, Chen R, Kim H, Ko HS, Dawson VL, Dawson TM. Kumar M, et al. Stem Cell Reports. 2020 Sep 8;15(3):629-645. doi: 10.1016/j.stemcr.2020.07.013. Epub 2020 Aug 13. Stem Cell Reports. 2020. PMID: 32795422 Free PMC article. - Regulation of Parkin E3 ubiquitin ligase activity.
Walden H, Martinez-Torres RJ. Walden H, et al. Cell Mol Life Sci. 2012 Sep;69(18):3053-67. doi: 10.1007/s00018-012-0978-5. Epub 2012 Apr 19. Cell Mol Life Sci. 2012. PMID: 22527713 Free PMC article. Review. - Analysis of neural subtypes reveals selective mitochondrial dysfunction in dopaminergic neurons from parkin mutants.
Burman JL, Yu S, Poole AC, Decal RB, Pallanck L. Burman JL, et al. Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10438-43. doi: 10.1073/pnas.1120688109. Epub 2012 Jun 12. Proc Natl Acad Sci U S A. 2012. PMID: 22691499 Free PMC article. - Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis.
Yu SB, Pekkurnaz G. Yu SB, et al. J Mol Biol. 2018 Oct 19;430(21):3922-3941. doi: 10.1016/j.jmb.2018.07.027. Epub 2018 Aug 5. J Mol Biol. 2018. PMID: 30089235 Free PMC article. Review.
References
- Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79:683–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases