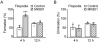Transient effects of anesthetics on dendritic spines and filopodia in the living mouse cortex - PubMed (original) (raw)
Transient effects of anesthetics on dendritic spines and filopodia in the living mouse cortex
Guang Yang et al. Anesthesiology. 2011 Oct.
Abstract
Background: Anesthetics are widely used to induce unconsciousness, pain relief, and immobility during surgery. It remains unclear whether the use of anesthetics has significant and long-lasting effects on synapse development and plasticity in the brain. To address this question, the authors examined the formation and elimination of dendritic spines, postsynaptic sites of excitatory synapses, in the developing mouse cortex during and after anesthetics exposure.
Methods: Transgenic mice expressing yellow fluorescence protein in layer 5 pyramidal neurons were used in this study. Mice at 1 month of age underwent ketamine-xylazine and isoflurane anesthesia over a period of hours. The elimination and formation rates of dendritic spines and filopodia, the precursors of spines, were followed over hours to days in the primary somatosensory cortex using transcranial two-photon microscopy. Four to five animals were examined under each experimental condition. Student t test and Mann-Whitney U test were used to analyze the data.
Results: Administration of either ketamine-xylazine or isoflurane rapidly altered dendritic filopodial dynamics but had no significant effects on spine dynamics. Ketamine-xylazine increased filopodial formation whereas isoflurane decreased filopodial elimination during 4 h of anesthesia. Both effects were transient and disappeared within a day after the animals woke up.
Conclusion: Studies suggest that exposure to anesthetics transiently affects the dynamics of dendritic filopodia but has no significant effect on dendritic spine development and plasticity in the cortex of 1-month-old mice.
Figures
Fig. 1
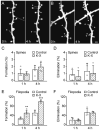
Administration of ketamine-xylazine rapidly increased the formation of dendritic filopodia but not spines over hours. A-B, In vivo time-lapse imaging of same dendritic segments over 4 h in the primary somatosensory cortex of 1-month-old animals that received no anesthesia (A) or ketamine-xylazine (K-X) anesthesia (B). Most dendritic spines on the same dendritic branches remained stable over 4 h whereas filopodia (asterisks) underwent rapid turnover. Scale bar, 2 μm. C-D, Percentage of newly formed (C) and eliminated (D) dendritic spines over 1 and 4 h. Administration of K-X did not alter spine dynamics. E-F, Percentage of newly formed (E) and eliminated (F) dendritic filopodia over 1 and 4 h. K-X anesthesia led to a rapid increase of filopodial formation but had no effect on filopodial elimination. Percentages were calculated as the number of spines/filopodia formed or eliminated divided by the number of pre-existing spines/filopodia. Each filled circle represents a single animal. Data are presented as mean ± S.D. ** P < 0.01; *** P < 0.001.
Fig. 2
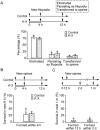
Ketamine-xylazine has no long lasting effects on the formation and elimination rates of dendritic spines and filopodia. A, Animals were under Ketamine-xylazine (K-X) anesthesia for the first 4 h and recovered for the next 8 h. B-C, Percentage of newly formed (B) and eliminated (C) dendritic spines over 12 h. D-E, Percentage of newly formed (D) and eliminated (E) dendritic filopodia over 12 h. There was no significant difference in spine or filopodial formation and elimination over 12 h between animals with and without K-X anesthesia. Percentages were calculated as the number of spines/filopodia formed or eliminated divided by the number of pre-existing spines/filopodia. Each filled circle represents a single animal. Data are presented as mean ± S.D.
Fig. 3
The majority of newly formed filopodia and spines do not persist. A, The percentage of new filopodia formed over the first 4 h that were eliminated, that persisted as filopodia, or that were transformed to spines over the next 8 h. The majority of filopodia were eliminated, a small percentage persisted, and less than 10% of filopodia were transformed to spines. There was no significant difference between filopodia formed with and without Ketamine-xylazine (K-X). B, Percentage of new spines persisting for 8 h. Less than half of new spines formed within the first 4 h persisted for the next 8 h. There was no significant difference between spines formed with and without K-X. C, Percentage of new spines persisting for 1 month. Less than 7% of new spines formed within 12 h or 2 days persisted over 1 month. Each filled circle represents a single animal. Data are presented as mean ± S.D.
Fig. 4
Systemic administration of MK801 mimics Ketamine-xylazine induced filopodial formation. A, Percentage of newly formed dendritic filopodia over 4 and 12 h. Animals were injected with MK801 after the first imaging session and re-imaged 4 and 12 h later. MK801 injection caused a rapid increase of filopodial formation over 4 but not 12 h. B, Percentage of eliminated dendritic filopodia over 4 and 12 h. MK801 had no significant effects on filopodial elimination. Percentages were calculated as the number of filopodia formed or eliminated divided by the number of pre-existing filopodia. Each filled circle represents a single animal. Data are presented as mean ± S.D. ***P < 0.001.
Fig. 5
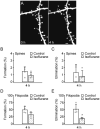
Administration of isoflurane affects the dynamics of dendritic filopodia but not spines. A, In vivo time-lapse imaging of the same dendritic segments over 4 h in 1-month-old, isoflurane anesthetized animals. Most dendritic spines remained stable over 4 h whereas filopodia (asterisks) underwent rapid turnover. Scale bar, 2 μm. B-C, Percentage of newly formed (B) and eliminated (C) dendritic spines over 4 h. Administration of isoflurane did not alter spine formation and elimination during this time period. D-E, Percentage of newly formed (D) and eliminated (E) dendritic filopodia over 4 h. Isoflurane anesthesia decreased the elimination of filopodia but had no significant effect on the formation of filopodia over 4 h. Percentages were calculated as the number of spines/filopodia formed or eliminated divided by the number of pre-existing spines/filopodia. Each filled circle represents a single animal. Data are presented as mean ± S.D. * P < 0.05.
Similar articles
- Longitudinal two-photon imaging in somatosensory cortex of behaving mice reveals dendritic spine formation enhancement by subchronic administration of low-dose ketamine.
Pryazhnikov E, Mugantseva E, Casarotto P, Kolikova J, Fred SM, Toptunov D, Afzalov R, Hotulainen P, Voikar V, Terry-Lorenzo R, Engel S, Kirov S, Castren E, Khiroug L. Pryazhnikov E, et al. Sci Rep. 2018 Apr 24;8(1):6464. doi: 10.1038/s41598-018-24933-8. Sci Rep. 2018. PMID: 29691465 Free PMC article. - The impact of development and sensory deprivation on dendritic protrusions in the mouse barrel cortex.
Chen CC, Bajnath A, Brumberg JC. Chen CC, et al. Cereb Cortex. 2015 Jun;25(6):1638-53. doi: 10.1093/cercor/bht415. Epub 2014 Jan 9. Cereb Cortex. 2015. PMID: 24408954 Free PMC article. - Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis.
Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Briner A, et al. Anesthesiology. 2010 Mar;112(3):546-56. doi: 10.1097/ALN.0b013e3181cd7942. Anesthesiology. 2010. PMID: 20124985 - Two-photon imaging of dendritic spine development in the mouse cortex.
Pan F, Gan WB. Pan F, et al. Dev Neurobiol. 2008 May;68(6):771-8. doi: 10.1002/dneu.20630. Dev Neurobiol. 2008. PMID: 18383548 Review. - Dendritic spine remodeling and plasticity under general anesthesia.
Granak S, Hoschl C, Ovsepian SV. Granak S, et al. Brain Struct Funct. 2021 Sep;226(7):2001-2017. doi: 10.1007/s00429-021-02308-6. Epub 2021 Jun 1. Brain Struct Funct. 2021. PMID: 34061250 Free PMC article. Review.
Cited by
- Post-anesthesia AMPA receptor potentiation prevents anesthesia-induced learning and synaptic deficits.
Huang L, Cichon J, Ninan I, Yang G. Huang L, et al. Sci Transl Med. 2016 Jun 22;8(344):344ra85. doi: 10.1126/scitranslmed.aaf7151. Sci Transl Med. 2016. PMID: 27334260 Free PMC article. - Propofol exposure in pregnant rats induces neurotoxicity and persistent learning deficit in the offspring.
Xiong M, Li J, Alhashem HM, Tilak V, Patel A, Pisklakov S, Siegel A, Ye JH, Bekker A. Xiong M, et al. Brain Sci. 2014 May 6;4(2):356-75. doi: 10.3390/brainsci4020356. Brain Sci. 2014. PMID: 24961766 Free PMC article. - Spine dynamics of PSD-95-deficient neurons in the visual cortex link silent synapses to structural cortical plasticity.
Yusifov R, Tippmann A, Staiger JF, Schlüter OM, Löwel S. Yusifov R, et al. Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2022701118. doi: 10.1073/pnas.2022701118. Proc Natl Acad Sci U S A. 2021. PMID: 33649238 Free PMC article. - Mechanistic insights into neurotoxicity induced by anesthetics in the developing brain.
Lei X, Guo Q, Zhang J. Lei X, et al. Int J Mol Sci. 2012;13(6):6772-6799. doi: 10.3390/ijms13066772. Epub 2012 Jun 4. Int J Mol Sci. 2012. PMID: 22837663 Free PMC article. Review. - Ketamine destabilizes growth of dendritic spines in developing hippocampal neurons in vitro via a Rho‑dependent mechanism.
Jiang S, Hao Z, Li X, Bo L, Zhang R, Wang Y, Duan X, Kang R, Huang L. Jiang S, et al. Mol Med Rep. 2018 Dec;18(6):5037-5043. doi: 10.3892/mmr.2018.9531. Epub 2018 Oct 2. Mol Med Rep. 2018. PMID: 30280188 Free PMC article.
References
- Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–12. - PubMed
- Anand KJ, Soriano SG. Anesthetic agents and the immature brain: Are these toxic or therapeutic? Anesthesiology. 2004;101:527–30. - PubMed
- Creeley CE, Olney JW. The young: Neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg. 2010;110:442–8. - PubMed
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS047325/NS/NINDS NIH HHS/United States
- R01 GM050686-12/GM/NIGMS NIH HHS/United States
- R01 NS047325-07/NS/NINDS NIH HHS/United States
- 3R01GM050686-12S1/GM/NIGMS NIH HHS/United States
- R01 GM050686/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources