Complex biotransformations catalyzed by radical S-adenosylmethionine enzymes - PubMed (original) (raw)
Review
Complex biotransformations catalyzed by radical S-adenosylmethionine enzymes
Qi Zhang et al. J Biol Chem. 2011.
Abstract
The radical S-adenosylmethionine (AdoMet) superfamily currently comprises thousands of proteins that participate in numerous biochemical processes across all kingdoms of life. These proteins share a common mechanism to generate a powerful 5'-deoxyadenosyl radical, which initiates a highly diverse array of biotransformations. Recent studies are beginning to reveal the role of radical AdoMet proteins in the catalysis of highly complex and chemically unusual transformations, e.g. the ThiC-catalyzed complex rearrangement reaction. The unique features and intriguing chemistries of these proteins thus demonstrate the remarkable versatility and sophistication of radical enzymology.
Figures
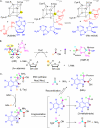
FIGURE 1.
Reactions of the radical AdoMet enzymes. A, binding of AdoMet to the [4Fe-4S] cluster and the reversible carbon–sulfur bond cleavage to produce the Ado radical. The unique iron for binding of AdoMet is highlighted in yellow. B, ThiC-catalyzed formation of HMP-P from AIR. The unexpected deuterium labeling patterns are highlighted in the yellow. C, NosL/NocL-catalyzed MIA biosynthesis.
FIGURE 2.
GTP-derived biosynthesis of Moco and deazapurine. A, role of MoaA and MoaC in Moco synthesis. B, key steps in queuosine biosynthesis. The reaction catalyzed by the radical AdoMet protein QueE is highlighted in yellow. CPH4, 6-carboxy-5,6,7,8-tetrahydropterin; CDG, 7-carboxy-7-deazaguanine.
FIGURE 3.
Radical-mediated methylation of aromatic heterocycles. A, RNA adenine methylation catalyzed by RlmN. B, C2′ methylation of
l
-Trp, possibly involving catalysis by the radical AdoMet methyltransferase TsrT (TsrM), proceeds with the net retention of the methyl group configuration.
Similar articles
- Radical-mediated enzymatic methylation: a tale of two SAMS.
Zhang Q, van der Donk WA, Liu W. Zhang Q, et al. Acc Chem Res. 2012 Apr 17;45(4):555-64. doi: 10.1021/ar200202c. Epub 2011 Nov 18. Acc Chem Res. 2012. PMID: 22097883 Free PMC article. Review. - Characterization of NocL involved in thiopeptide nocathiacin I biosynthesis: a [4Fe-4S] cluster and the catalysis of a radical S-adenosylmethionine enzyme.
Zhang Q, Chen D, Lin J, Liao R, Tong W, Xu Z, Liu W. Zhang Q, et al. J Biol Chem. 2011 Jun 17;286(24):21287-94. doi: 10.1074/jbc.M111.224832. Epub 2011 Mar 29. J Biol Chem. 2011. PMID: 21454624 Free PMC article. - Radicals from S-adenosylmethionine and their application to biosynthesis.
Roach PL. Roach PL. Curr Opin Chem Biol. 2011 Apr;15(2):267-75. doi: 10.1016/j.cbpa.2010.11.015. Epub 2010 Dec 13. Curr Opin Chem Biol. 2011. PMID: 21159543 Review. - Radical mechanisms in adenosylmethionine- and adenosylcobalamin-dependent enzymatic reactions.
Frey PA, Reed GH. Frey PA, et al. Arch Biochem Biophys. 2000 Oct 1;382(1):6-14. doi: 10.1006/abbi.2000.2010. Arch Biochem Biophys. 2000. PMID: 11051091 Review. - The thiamine biosynthetic enzyme ThiC catalyzes multiple turnovers and is inhibited by S-adenosylmethionine (AdoMet) metabolites.
Palmer LD, Downs DM. Palmer LD, et al. J Biol Chem. 2013 Oct 18;288(42):30693-30699. doi: 10.1074/jbc.M113.500280. Epub 2013 Sep 6. J Biol Chem. 2013. PMID: 24014032 Free PMC article.
Cited by
- Sandacrabins - Structurally Unique Antiviral RNA Polymerase Inhibitors from a Rare Myxobacterium.
Bader CD, Panter F, Garcia R, Tchesnokov EP, Haid S, Walt C, Spröer C, Kiefer AF, Götte M, Overmann J, Pietschmann T, Müller R. Bader CD, et al. Chemistry. 2022 Feb 21;28(10):e202104484. doi: 10.1002/chem.202104484. Epub 2022 Jan 22. Chemistry. 2022. PMID: 34990513 Free PMC article. - Viperin: An ancient radical SAM enzyme finds its place in modern cellular metabolism and innate immunity.
Ghosh S, Marsh ENG. Ghosh S, et al. J Biol Chem. 2020 Aug 14;295(33):11513-11528. doi: 10.1074/jbc.REV120.012784. Epub 2020 Jun 16. J Biol Chem. 2020. PMID: 32546482 Free PMC article. Review. - Discovery of novel bacterial queuine salvage enzymes and pathways in human pathogens.
Yuan Y, Zallot R, Grove TL, Payan DJ, Martin-Verstraete I, Šepić S, Balamkundu S, Neelakandan R, Gadi VK, Liu CF, Swairjo MA, Dedon PC, Almo SC, Gerlt JA, de Crécy-Lagard V. Yuan Y, et al. Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):19126-19135. doi: 10.1073/pnas.1909604116. Epub 2019 Sep 3. Proc Natl Acad Sci U S A. 2019. PMID: 31481610 Free PMC article. - Recent advances in radical SAM enzymology: new structures and mechanisms.
Wang J, Woldring RP, Román-Meléndez GD, McClain AM, Alzua BR, Marsh EN. Wang J, et al. ACS Chem Biol. 2014 Sep 19;9(9):1929-38. doi: 10.1021/cb5004674. Epub 2014 Jul 16. ACS Chem Biol. 2014. PMID: 25009947 Free PMC article. Review. - C-H methylation of heteroarenes inspired by radical SAM methyl transferase.
Gui J, Zhou Q, Pan CM, Yabe Y, Burns AC, Collins MR, Ornelas MA, Ishihara Y, Baran PS. Gui J, et al. J Am Chem Soc. 2014 Apr 2;136(13):4853-6. doi: 10.1021/ja5007838. Epub 2014 Mar 21. J Am Chem Soc. 2014. PMID: 24611732 Free PMC article.
References
- Frey P. A., Hegeman A. D., Reed G. H. (2006) Chem. Rev. 106, 3302–3316 - PubMed
- Renaud P., Sibi M. P. (eds) (2001) Radicals in Organic Synthesis, Wiley-VCH, Weinheim, Germany
- Marsh E. N., Patwardhan A., Huhta M. S. (2004) Bioorg. Chem. 32, 326–340 - PubMed
- Frey P. A., Hegeman A. D., Ruzicka F. J. (2008) Crit. Rev. Biochem. Mol. Biol. 43, 63–88 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases