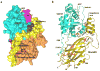The 'invisible hand': regulation of RHO GTPases by RHOGDIs - PubMed (original) (raw)
Review
The 'invisible hand': regulation of RHO GTPases by RHOGDIs
Rafael Garcia-Mata et al. Nat Rev Mol Cell Biol. 2011.
Abstract
The 'invisible hand' is a term originally coined by Adam Smith in The Theory of Moral Sentiments to describe the forces of self-interest, competition and supply and demand that regulate the resources in society. This metaphor continues to be used by economists to describe the self-regulating nature of a market economy. The same metaphor can be used to describe the RHO-specific guanine nucleotide dissociation inhibitor (RHOGDI) family, which operates in the background, as an invisible hand, using similar forces to regulate the RHO GTPase cycle.
Figures
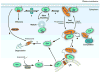
Figure 1. The RHOGDI cycle
(a) Newly synthesized RHO family GTPases are geranylgeranylated and then post-translationally modified by the protease Ras-converting enzyme 1 (RCE1) and by isoprenylcysteine carboxyl methyltransferase (ICMT) at the cytoplasmic face of the endoplasmic reticulum (ER). (b) After geranylgeranylation, RHO proteins associate with RHO specific guanine nucleotide dissociation inhibitors (RHOGDIs), which sequester them in the cytosol and protect them from degradation. (c) Free prenylated cytosolic RHO GTPases are unstable and are rapidly degraded by the proteasome. (d) Several RHO GTPases can associate with RHOGDI and compete for its binding. Overexpression of a GTPase can displace the endogenous RHO proteins from RHOGDI targeting them for degradation. (e) The rate of cycling of the RHOGDI -RHO GTPase complex between the cytosol and the membrane can be regulated by post-translational modifications on both the RHO GTPases and the RHOGDI, which modulate the affinity of the interaction. A slower pathway for recycling RHO proteins through vesicle trafficking has also been postulated. (f) Once at the membrane, the RHO GTPases can be activated by guanine nucleotide exchange factors (GEFs) and bind to downstream effectors. Following inactivation by GTPase-activating proteins (GAPs), RHO GTPases are extracted from the membrane by RHOGDI. (g) Active RHOA can also be targeted for degradation by the ubiquitin ligase SMAD ubiquitylation regulatory factor 1 (Smurf1). GGTase, geranylgeranyl transferase.
Figure 2. Structure of the RHOGDI–RHO GTPase complex
(A) Space filling model showing CDC42 in complex with RHO-specific guanine nucleotide dissociation inhibitor 1 (RHOGDI1). The domains that participate in the interaction are highlighted and labelled on the struucture. (B) Cartoon representation of the crystal structure of prenylated CDC42 in complex with RHOGDI . The phosphorylated residues are shown in magenta, CDC42 is shown in green, and RHOGDI1 is shown in gold. These structures are reproduced from the
NCBI Molecular Modeling Database
(ID:
1275
). C, carboxyl terminus; GG, geranylgeranyl; N, amino terminus; PBR, Poly Basic Region.
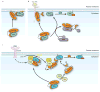
Figure 3. Mechanisms of regulation of the RHOGDI-RHOGTPase interaction
a | Release by lipids: The presence of acidic phospholipids can promote the release of RHOGTPases from RHO-specific guanine nucleotide dissociation inhibitors (RHOGDIs). Phospholipids mediate a partial opening of the complex that exposes the GTPases to RHO-specific guanine nucleotide exchange factors (RHOGEFs) or other dissociation factors such as the ones described in b. b | Release by protein–protein interactions: p75 neurotrophin receptor (p75NTR) and ezrin, radixin and moesin (ERM) proteins interact with RHOGDIs and facilitate the release of RHOA, which can then be activated by specific RHOGEFs. The interaction of p75NTR with RHOGDIs is enhanced by myelin derived proteins such as myelin-associated glycoprotein (MAG) and neurite outgrowth inhibitor (NOGO). ERM proteins also need to be activated to interact with RHOGDI. c | Release by phosphorylation. Phosphorylation of RHOGDI Ser, Thr and Tyr residues promote the release of RHO GTPases. Depending on the residues phosphorylated, this release can be specific for a single RHO GTPase, or affect multiple RHO GTPases simultaneously (Table 1). For example p21-activated kinase (PAK)- or FER-mediated phosphorylation promote the specific release of RAC1, but not RHOA or CDC42. Some kinases act in concert to target the RAC1–RHODI complex to the membrane and regulate the local release of RAC1 at specific site on the membrane, where it is subsequently activated by RHOGEFs. Diacylglycerol kinase-ζ (DGKζ) forms a complex with PAK, RAC1 and RHOGDI. In response to platelet derived growth factor (PDGF), DGKζ stimulates the production of phosphatidic acid (PA), which induces PAK activity. Active PAK then phosphorylates RHOGDI and releases RAC1 for activation. Positively charged phospholipids are indicated in blue, negatively charged phopholipids in red. DAG, diacylglycerol; PDGFR, PDGF receptor.
Similar articles
- Uncoupling of inhibitory and shuttling functions of rho GDP dissociation inhibitors.
Dransart E, Morin A, Cherfils J, Olofsson B. Dransart E, et al. J Biol Chem. 2005 Feb 11;280(6):4674-83. doi: 10.1074/jbc.M409741200. Epub 2004 Oct 28. J Biol Chem. 2005. PMID: 15513926 - A Complete Survey of RhoGDI Targets Reveals Novel Interactions with Atypical Small GTPases.
Ahmad Mokhtar AMB, Ahmed SBM, Darling NJ, Harris M, Mott HR, Owen D. Ahmad Mokhtar AMB, et al. Biochemistry. 2021 May 18;60(19):1533-1551. doi: 10.1021/acs.biochem.1c00120. Epub 2021 Apr 29. Biochemistry. 2021. PMID: 33913706 Free PMC article. - RhoGDI SUMOylation at Lys-138 increases its binding activity to Rho GTPase and its inhibiting cancer cell motility.
Yu J, Zhang D, Liu J, Li J, Yu Y, Wu XR, Huang C. Yu J, et al. J Biol Chem. 2012 Apr 20;287(17):13752-60. doi: 10.1074/jbc.M111.337469. Epub 2012 Mar 5. J Biol Chem. 2012. PMID: 22393046 Free PMC article. - RhoGDI-3, a promising system to investigate the regulatory function of rhoGDIs: uncoupling of inhibitory and shuttling functions of rhoGDIs.
Dransart E, Morin A, Cherfils J, Olofsson B. Dransart E, et al. Biochem Soc Trans. 2005 Aug;33(Pt 4):623-6. doi: 10.1042/BST0330623. Biochem Soc Trans. 2005. PMID: 16042558 Review. - RhoGDIs revisited: novel roles in Rho regulation.
Dransart E, Olofsson B, Cherfils J. Dransart E, et al. Traffic. 2005 Nov;6(11):957-66. doi: 10.1111/j.1600-0854.2005.00335.x. Traffic. 2005. PMID: 16190977 Review.
Cited by
- Proteomic analysis in lupus mice identifies Coronin-1A as a potential biomarker for lupus nephritis.
Nicolaou O, Sokratous K, Makowska Z, Morell M, De Groof A, Montigny P, Hadjisavvas A, Michailidou K, Oulas A, Spyrou GM, Demetriou C, Alarcón-Riquelme ME, Psarellis S, Kousios A, Lauwerys B, Kyriacou K. Nicolaou O, et al. Arthritis Res Ther. 2020 Jun 18;22(1):147. doi: 10.1186/s13075-020-02236-6. Arthritis Res Ther. 2020. PMID: 32552896 Free PMC article. - ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling.
Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, Beck BB, Gribouval O, Zhou W, Diaz KA, Natarajan S, Wiggins RC, Lovric S, Chernin G, Schoeb DS, Ovunc B, Frishberg Y, Soliman NA, Fathy HM, Goebel H, Hoefele J, Weber LT, Innis JW, Faul C, Han Z, Washburn J, Antignac C, Levy S, Otto EA, Hildebrandt F. Gee HY, et al. J Clin Invest. 2013 Aug;123(8):3243-53. doi: 10.1172/JCI69134. Epub 2013 Jul 8. J Clin Invest. 2013. PMID: 23867502 Free PMC article. - ATPase and GTPase Tangos Drive Intracellular Protein Transport.
Shan SO. Shan SO. Trends Biochem Sci. 2016 Dec;41(12):1050-1060. doi: 10.1016/j.tibs.2016.08.012. Epub 2016 Sep 19. Trends Biochem Sci. 2016. PMID: 27658684 Free PMC article. Review. - ARF1 and ARF6 regulate recycling of GRASP/Tamalin and the Rac1-GEF Dock180 during HGF-induced Rac1 activation.
Koubek EJ, Santy LC. Koubek EJ, et al. Small GTPases. 2018 May 4;9(3):242-259. doi: 10.1080/21541248.2016.1219186. Epub 2016 Aug 26. Small GTPases. 2018. PMID: 27562622 Free PMC article. - Activation of RhoC by regulatory ubiquitination is mediated by LNX1 and suppressed by LIS1.
Kholmanskikh S, Singh S, Ross ME. Kholmanskikh S, et al. Sci Rep. 2022 Oct 3;12(1):16493. doi: 10.1038/s41598-022-19740-1. Sci Rep. 2022. PMID: 36192543 Free PMC article.
References
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–63. - PubMed
- Fukumoto Y, et al. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990;5:1321–8. - PubMed
- Leonard D, et al. The identification and characterization of a GDP-dissociation inhibitor (GDI) for the CDC42Hs protein. J Biol Chem. 1992;267:22860–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL080166/HL/NHLBI NIH HHS/United States
- R01 GM029860-29A1/GM/NIGMS NIH HHS/United States
- P01 HL080166-05/HL/NHLBI NIH HHS/United States
- R01 GM029860/GM/NIGMS NIH HHS/United States
- P01 HL080166/HL/NHLBI NIH HHS/United States
- GM029860/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources