Activation of Akt by the bacterial inositol phosphatase, SopB, is wortmannin insensitive - PubMed (original) (raw)
Activation of Akt by the bacterial inositol phosphatase, SopB, is wortmannin insensitive
Kendal G Cooper et al. PLoS One. 2011.
Abstract
Salmonella enterica uses effector proteins translocated by a Type III Secretion System to invade epithelial cells. One of the invasion-associated effectors, SopB, is an inositol phosphatase that mediates sustained activation of the pro-survival kinase Akt in infected cells. Canonical activation of Akt involves membrane translocation and phosphorylation and is dependent on phosphatidyl inositide 3 kinase (PI3K). Here we have investigated these two distinct processes in Salmonella infected HeLa cells. Firstly, we found that SopB-dependent membrane translocation and phosphorylation of Akt are insensitive to the PI3K inhibitor wortmannin. Similarly, depletion of the PI3K regulatory subunits p85α and p85ß by RNAi had no inhibitory effect on SopB-dependent Akt phosphorylation. Nevertheless, SopB-dependent phosphorylation does depend on the Akt kinases, PDK1 and rictor-mTOR. Membrane translocation assays revealed a dependence on SopB for Akt recruitment to Salmonella ruffles and suggest that this is mediated by phosphoinositide (3,4) P(2) rather than phosphoinositide (3,4,5) P(3). Altogether these data demonstrate that Salmonella activates Akt via a wortmannin insensitive mechanism that is likely a class I PI3K-independent process that incorporates some essential elements of the canonical pathway.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
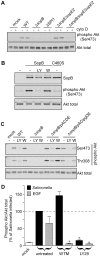
Figure 1. SopB–dependent Akt phosphorylation in epithelial cells is wortmannin insensitive.
(A) HeLa cells were infected with Salmonella, either WT or the indicated mutants, for 20 min. Monolayers were then solubilized in sample buffer and processed for immunoblotting using rabbit polyclonal antibodies to detect phospho Akt (Ser473) and total Akt. Cytochalasin D (cyto D: 1 µg/ml) treated cells were incubated with drug prior to and throughout the infection. (B) HeLa cells were transfected with plasmid expressing 6His-SopB or 6His-SopB C460S for 18 hr. Monolayers were solubilized and processed for immunoblotting using antibodies against SopB, phospho Akt (Ser473) and total Akt. Where indicated, LY294002 (LY: 50 µM) or wortmannin (W:100 nM) were added for 40 min prior to sample collection. (C & D) HeLa cells were treated with EGF (50 ng/ml) for 2 min or infected with Salmonella for 30 min then solubilized and processed for immunoblotting (C) or ELISA (D). To compare the activities of SopB and IpgD the Δ_sopB_ strain was complemented with plasmids pACDE or pACipgDE, respectively. Where indicated cells were pretreated with either LY294002 (LY29: 50 µM) or wortmannin (WTM: 100 nM) for 30 min preceding infection and inhibitor was maintained in the media for all subsequent incubations. Immunoblots are representative from three independent experiments. ELISA data represent means ± SD from three independent experiments (* P<0.05, significantly different from untreated).
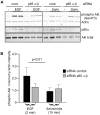
Figure 2. The class I PI3K regulatory subunits p85α and p85ß are not required for SopB-mediated Akt phosphorylation.
HeLa cells were transfected with siRNAs, specific for p85α and p85ß, for 72 hr then either treated with EGF or infected with Salmonella WT. For siRNA control siRNA specific for AKT3 was used (cont.). Monolayers were then solubilized in sample buffer and processed for immunoblotting using antibodies to detect phospho Akt (Ser473), total Akt or p85α. (A) Representative immunoblot showing p85α knockdown efficiacy and effect on Akt phosphorylation in infected or EGF treated cells. (B) Quantification of Akt phosphorylation estimated by densitometry. Shown are the means ± SD from three independent experiments.
Figure 3. PI3K/Akt inhibitors differentially affect _Salmonella_-induced and EGF-induced Akt phosphorylation in epithelial cells.
HeLa cells were pretreated with PI3K/Akt inhibitors for 30 min and then infected with Salmonella for 30 min (A) or treated with EGF (50 ng/ml) for 2 min (B). Monolayers were then solubilized in sample buffer and processed for immunoblotting using antibodies to detect phospho Akt (Ser473) and total Akt. Inhibitors used were; Wortmanin (WTM:100 nM), LY294002 (LY29: 50 µM), SH-6 (20 µM, 10 µM), TCN (20 µM, 10 µM), Akti-1/2 (0.1 µM, 0.05 µM) and AIX (10 µM, 5 µM). The graphs below each panel show the quantification of Akt phosphorylation estimated by densitometry. Shown are the means ± SD from three independent experiments (* P<0.05, significantly different from untreated).
Figure 4. Both PDK1 and rictor are required for _Salmonella_-induced Akt phosphorylation.
A and B. HeLa cells were transfected with the indicated SMART pool siRNA for 48 hr then infected with Salmonella WT or Δ_sopB_. For siRNA control SMART pool siRNA specific for AKT3 was used (cont.). After 30 min, monolayers were solubilized and processed for immunoblotting. Antibodies were used to detect phospho-Akt (Ser473 or Thr308), total Akt, PDK1, raptor, rictor or actin. Gray bars underneath the individual panels highlight the efficiency of each siRNA knockdown.
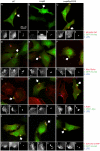
Figure 5. Akt regulators accumulate in Salmonella induced ruffles.
HeLa cells were transfected with plasmids encoding epitope-tagged proteins as indicated. After 20 hr they were infected with Salmonella for 30 min, then fixed and processed for immunofluorescence microscopy. GFP-HA-Akt transfected cells were stained for Salmonella LPS (Cy5) and phospho Akt Ser473 (AF568: A–C), Myc (AF568: D–F) or 3×FLAG (AF568: J–L). Cells expressing PDK1-Myc (G–I) were stained for LPS (Cy5), Myc (AF488) and actin filaments (phalloidin-AF568) to reveal the ruffles.
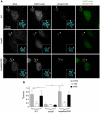
Figure 6. Accumulation of phospho Akt in _Salmonella_-induced ruffles is wortmannin insensitive.
HeLa cells expressing EGFP-mAkt were infected with Salmonella for 20 min then fixed and processed for immunofluorescence microscopy. Cells were doubly stained for plasma membrane (Cy5-WGA) and phospho Akt Ser473 (AF568). (A) Representative images with ruffles outlined to show phospho Akt in ruffles induced by WT Salmonella. In comparison, phospho Akt levels are much lower in ruffles induced by the Δ_sopB_ mutant, unless the mutant is complemented in trans with sopB (pACDE). (B) Semi-quantitative analysis of phospho Akt (Ser473) levels (R pAkt/Akt) in membrane ruffles. Where indicated, cells were pretreated with wortmannin (WTM: 100 nM) or LY294002 (LY29: 50 µM) for 30 min prior to infection and maintained throughout. Data are the means ± SD from three independent experiments (* P<0.05).
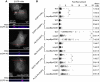
Figure 7. Enrichment of PH domain lipid-binding probes in _Salmonella_-induced ruffles.
HeLa cells transfected with plasmids encoding EGFP-fusions to full length Akt (EGFP-mAkt) or isolated PH domains as indicated were infected with Salmonella for 30 min, then fixed and processed for immunofluorescence microscopy. Cells were stained for plasma membrane (Cy5-WGA) and Salmonella LPS (AF568). (A) Representative images to show EGFP-mAkt accumulation in ruffles induced by Salmonella WT, the Δ_sopB_ mutant or Δ_sopB_ complemented in trans with sopB (pACDE). Orthogonal sections show WGA (grayscale) and EGFP-Akt (grayscale converted to a heatmap using the “FIRE” look up table of ImageJ) corresponding to the red lines on the projections. (B) Analysis of GFP fusion enrichment in membrane ruffles. Shown is combined data from three independent experiments. Each dot represents one ruffle. P values were obtained by ANOVA and Tukey's post hoc analysis (* P<0.05).
Similar articles
- Multiple host kinases contribute to Akt activation during Salmonella infection.
Roppenser B, Kwon H, Canadien V, Xu R, Devreotes PN, Grinstein S, Brumell JH. Roppenser B, et al. PLoS One. 2013 Aug 22;8(8):e71015. doi: 10.1371/journal.pone.0071015. eCollection 2013. PLoS One. 2013. PMID: 23990921 Free PMC article. - A second wave of Salmonella T3SS1 activity prolongs the lifespan of infected epithelial cells.
Finn CE, Chong A, Cooper KG, Starr T, Steele-Mortimer O. Finn CE, et al. PLoS Pathog. 2017 Apr 20;13(4):e1006354. doi: 10.1371/journal.ppat.1006354. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28426838 Free PMC article. - SopB activates the Akt-YAP pathway to promote Salmonella survival within B cells.
García-Gil A, Galán-Enríquez CS, Pérez-López A, Nava P, Alpuche-Aranda C, Ortiz-Navarrete V. García-Gil A, et al. Virulence. 2018;9(1):1390-1402. doi: 10.1080/21505594.2018.1509664. Virulence. 2018. PMID: 30103648 Free PMC article. - The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt.
Knodler LA, Finlay BB, Steele-Mortimer O. Knodler LA, et al. J Biol Chem. 2005 Mar 11;280(10):9058-64. doi: 10.1074/jbc.M412588200. Epub 2005 Jan 10. J Biol Chem. 2005. PMID: 15642738 - PI3 kinase directly phosphorylates Akt1/2 at Ser473/474 in the insulin signal transduction pathway.
Tsuchiya A, Kanno T, Nishizaki T. Tsuchiya A, et al. J Endocrinol. 2013 Nov 28;220(1):49-59. doi: 10.1530/JOE-13-0172. Print 2014 Jan. J Endocrinol. 2013. PMID: 24169049 Free PMC article.
Cited by
- Salmonella Typhimurium disrupts Sirt1/AMPK checkpoint control of mTOR to impair autophagy.
Ganesan R, Hos NJ, Gutierrez S, Fischer J, Stepek JM, Daglidu E, Krönke M, Robinson N. Ganesan R, et al. PLoS Pathog. 2017 Feb 13;13(2):e1006227. doi: 10.1371/journal.ppat.1006227. eCollection 2017 Feb. PLoS Pathog. 2017. PMID: 28192515 Free PMC article. - SMER28 Attenuates PI3K/mTOR Signaling by Direct Inhibition of PI3K p110 Delta.
Kirchenwitz M, Stahnke S, Prettin S, Borowiak M, Menke L, Sieben C, Birchmeier C, Rottner K, Stradal TEB, Steffen A. Kirchenwitz M, et al. Cells. 2022 May 16;11(10):1648. doi: 10.3390/cells11101648. Cells. 2022. PMID: 35626685 Free PMC article. - Salmonella Exhibit Altered Cellular Localization in the Presence of HLA-B27 and Codistribute with Endo-Reticular Membrane.
Kriston-Vizi J, Lenart I, Iwawaki T, Gould K, Nesbeth D, Powis SJ, Antoniou AN. Kriston-Vizi J, et al. J Immunol Res. 2022 Sep 16;2022:9493019. doi: 10.1155/2022/9493019. eCollection 2022. J Immunol Res. 2022. PMID: 36157878 Free PMC article. - Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages.
Owen KA, Meyer CB, Bouton AH, Casanova JE. Owen KA, et al. PLoS Pathog. 2014 Jun 5;10(6):e1004159. doi: 10.1371/journal.ppat.1004159. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24901456 Free PMC article. - Infection biology of Salmonella enterica.
Han J, Aljahdali N, Zhao S, Tang H, Harbottle H, Hoffmann M, Frye JG, Foley SL. Han J, et al. EcoSal Plus. 2024 Dec 12;12(1):eesp00012023. doi: 10.1128/ecosalplus.esp-0001-2023. Epub 2024 Jan 4. EcoSal Plus. 2024. PMID: 38415623 Free PMC article. Review.
References
- Zhou D, Galan J. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 2001;3:1293–1298. - PubMed
- Terebiznik MR, Vieira OV, Marcus SL, Slade A, Yip CM, et al. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol. 2002;4:766–773. - PubMed
- Hernandez LD, Hueffer K, Wenk MR, Galan JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 2004;304:1805–1807. - PubMed
- Drecktrah D, Knodler LA, Galbraith K, Steele-Mortimer O. The Salmonella SPI1 effector SopB stimulates nitric oxide production long after invasion. Cell Microbiol. 2005;7:105–113. - PubMed
- Steele-Mortimer O, Knodler LA, Marcus SL, Scheid MP, Goh B, et al. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J Biol Chem. 2000;275:37718–37724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous