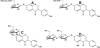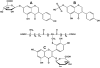The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems - PubMed (original) (raw)
Review
The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems
Stephen Barnes et al. Food Funct. 2011 May.
Abstract
Polyphenols in dietary and botanical matrices are usually present as simple and complex O-glycosides. In fermented dietary materials, the glycosidic moiety is removed and accompanied in some cases by more complex changes to the polyphenol. As for most xenobiotics, polyphenols undergo phase II conjugation in the intestinal wall during their absorption from the gut. In contrast, a few polyphenols, such as puerarin in the kudzu vine, are C-glycosides and are stable in the gut and during absorption, distribution and excretion. Large bowel bacteria reduce polyphenol aglycones, causing opening of the heterocyclic B-ring and ring cleavage. The products are mostly absorbed and enter the bloodstream. Phase I and II metabolism events occur in the intestine and the liver - most polyphenols predominantly circulate as β-glucuronides and sulfate esters with very little as the aglycones, the presumed active forms. In addition, metabolism can occur in non-hepatic tissues and cells including breast tumor cells that have variable amounts of cytochrome P450s, sulfatase and sulfotransferase activities. Inflammatory cells produce chemical oxidants (HOCl, HOBr, ONO(2)(-)) that will react with polyphenols. The isoflavones daidzein and genistein and the flavonol quercetin form mono- and dichlorinated products in reaction with HOCl. Genistein is converted to 3'-nitrogenistein in the lung tissue of lipopolysaccharide-treated rats. Whereas polyphenols that can be converted to quinones or epoxides react with glutathione (GSH) to form adducts, chlorinated isoflavones do not react with GSH; instead, they are converted to β-glucuronides and are excreted in bile. Analysis of polyphenols and their metabolites is routinely carried out with great sensitivity, specificity and quantification by LC-tandem mass spectrometry. Critical questions about the absorption and tissue uptake of complex polyphenols such as the proanthocyanins can be answered by labeling these polyphenols with (14)C-sucrose in plant cell culture and then purifying them for use in animal experiments. The (14)C signature is quantified using accelerator mass spectrometry, a technique capable of detecting one (14)C atom in 10(15) carbon atoms. This permits the study of the penetration of the polyphenols into the interstitial fluid, the fluid that is actually in contact with non-vascular cells.
Figures
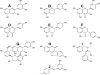
Figure 1. Examples of polyphenol structures by class
A, Anthocyanins (delphinidin); B, flavanols (epi-catechin); C, flavonols (quercetin); D, flavone (naringenin); E, flavanones (naringenin); F, isoflavones (genistein); G, proanthocyanidins (proanthocyanidin B1); H, coumestanes (coumesterol); I, lignans (enterodiol); and J, stilbenoids (resveratrol).
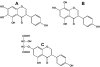
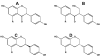
Figure 2. Glycoside esters of isoflavones
A, 6″-_O_-malonyl-7-_O_-β-D-glucoside of genistein (soy); B, 6″-_O_-acetyl-7-_O_-β-D-glucoside of genistein (soy); C, 7-_O_-β-D-glucosylglucoside of genistein (Apios Americana); D, 8-_C_-glycoside of daidzein (Kudzu root, Puerariae lobata).
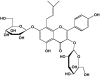
Figure 3. Structure of the prenylated flavonoid, icariin, from horny goat weed
Icariin is 8-prenyl kaempferol 3,7-diglucoside.
Figure 4. Modified isoflavones in fermented soy sauces
A; 6-hydroxygenistein; B, 8-hydroxygenistein; C, genistein-7-tartaric acid ether.
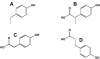
Figure 5. Reduced metabolites of daidzein and genistein
A, dihydrodaidzein; B, O-desmethylangolensin; C, R-(+)equol; D, S-(−)equol.
Figure 6. Ring opened metabolites of flavonoids and isoflavonoids
A, p-ethyl phenol; B, 2-(4-hydroxyphenyl)-propionic acid; C, 3-(4-hydroxyphenyl)-propionic acid; D, phenylacetic acid.
Figure 7. Conjugated flavonoids and isoflavonoids
A; genistein-7-O-β-D-glucuronide; B, genistein-7-sulfonate; C, 3′-glutathionyl quercetin 3-D-glucuronide.
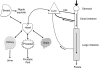
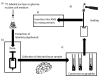
Figure 8. Distribution of polyphenols in the body
Ingested polyphenols are mostly taken up after hydrolysis by passive diffusion from the small and large intestines. Bacterial metabolites are formed in the colon. The mesenteric blood supply takes them back to the liver. Hepatic extraction is efficient and conjugated polyphenols are secreted into the bile. Polyphenols and their metabolites circulate at sub-μM concentrations in the blood that perfuses the organs. Small amounts pass the blood-brain barrier. Concentrations in the μM range are found in nipple aspirate and prostatic fluid. The kidney readily filters polyphenols and their metabolites and active secretion may also occur. Urine concentrations are often > 20 μM for the bioavailable polyphenols (isoflavones), but are much lower for the complex polyphenols (proanthocyanidins) which are poorly absorbed.
Figure 9. Design of an experiment using accelerator mass spectrometry
(A) Plant cells are incubated with 14CO or 14 2 C-labeled sucrose and the polyphenols extracted and chromatographically purified; (B) a small dose of 14C-polyphenol is administered to the animal or clinical subject; (C) the biological fluid or tissue is recovered and converted to graphite; (D) the graphitic material is converted to a plug; and (E) the plug is inserted into the AMS for analysis
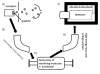
Figure 10. A cartoon depicting a typical AMS experiment
Ionization of the sample in ion source (A), is followed by mass separation (B), destruction of interfering molecules (C), more mass and energy analysis (D), and finally detection in a gas-ionization detector (E).
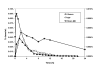
Figure 11. Use of accelerator mass spectrometry to track the distribution of 14C-labeled polyphenols in tissues
A 14C-preparation of proanthocyanidins (50 nCi) was administered to adult rats by gavage. Blood, interstitial fluid and brain microdialysate were collected from unanesthetized, free-living rats implanted with in-dwelling cannulas to recover these fluids.
Similar articles
- Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry.
Doerge DR, Chang HC, Churchwell MI, Holder CL. Doerge DR, et al. Drug Metab Dispos. 2000 Mar;28(3):298-307. Drug Metab Dispos. 2000. PMID: 10681374 Clinical Trial. - Bioavailability of the glucuronide and sulfate conjugates of genistein and daidzein in breast cancer resistance protein 1 knockout mice.
Álvarez AI, Vallejo F, Barrera B, Merino G, Prieto JG, Tomás-Barberán F, Espín JC. Álvarez AI, et al. Drug Metab Dispos. 2011 Nov;39(11):2008-12. doi: 10.1124/dmd.111.040881. Epub 2011 Aug 9. Drug Metab Dispos. 2011. PMID: 21828252 - Flavonoid metabolism: the interaction of metabolites and gut microbiota.
Murota K, Nakamura Y, Uehara M. Murota K, et al. Biosci Biotechnol Biochem. 2018 Apr;82(4):600-610. doi: 10.1080/09168451.2018.1444467. Epub 2018 Mar 5. Biosci Biotechnol Biochem. 2018. PMID: 29504827 Review. - Analysis of isoflavones in foods and dietary supplements.
Delmonte P, Rader JI. Delmonte P, et al. J AOAC Int. 2006 Jul-Aug;89(4):1138-46. J AOAC Int. 2006. PMID: 16915857 Review.
Cited by
- Study on biotransformation and absorption of genistin based on fecal microbiota and Caco-2 cell.
Li Z, Wang Y, Wang Z, Wu D, Zhao Y, Gong X, Jiang Q, Xia C. Li Z, et al. Front Pharmacol. 2024 Oct 9;15:1437020. doi: 10.3389/fphar.2024.1437020. eCollection 2024. Front Pharmacol. 2024. PMID: 39444613 Free PMC article. - The Mediterranean Diet, Its Microbiome Connections, and Cardiovascular Health: A Narrative Review.
Abrignani V, Salvo A, Pacinella G, Tuttolomondo A. Abrignani V, et al. Int J Mol Sci. 2024 Apr 30;25(9):4942. doi: 10.3390/ijms25094942. Int J Mol Sci. 2024. PMID: 38732161 Free PMC article. Review. - Influence of malting procedure on the isoflavonoid content of soybeans.
Gasiński A, Mikulski D, Kłosowski G, Kawa-Rygielska J. Gasiński A, et al. Sci Rep. 2024 Mar 26;14(1):7184. doi: 10.1038/s41598-024-57914-1. Sci Rep. 2024. PMID: 38532039 Free PMC article. - Heat shock increases the anti-inflammatory and anti-obesity activity of soybean by increasing polyphenol, antioxidant and aglycon form isoflavones.
Khatun S, Kim T, Mollah MMI. Khatun S, et al. Heliyon. 2023 Nov 8;9(11):e21944. doi: 10.1016/j.heliyon.2023.e21944. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034630 Free PMC article. - Effects of fruits and vegetables on gut microbiota in a mouse model of metabolic syndrome induced by high-fat diet.
Yu C, Guo C, Geng X, Yao Y, Guo J, Zhang Y, Zhang J, Mi S. Yu C, et al. Food Sci Nutr. 2022 Nov 5;11(2):794-805. doi: 10.1002/fsn3.3114. eCollection 2023 Feb. Food Sci Nutr. 2022. PMID: 36789067 Free PMC article.
References
- Kudou S, Fleury Y, Welti D, Magnolato D, Uchida T, Kitamura K, Okubo K. Malonyl isoflavone glycosides in soybean seeds (Glycine max MERRILL) Agric. Biol. Chem. 1991;55:2227–2233.
- Barnes S, Kirk M, Coward L. Isoflavones and their conjugates in soy foods: extraction conditions and analysis by HPLC-mass spectrometry. J. Agric. Food Chem. 1994;42:2466–2474.
- Barnes S, Wang C-C, Kirk M, Smith-Johnson M, Coward L, Barnes NC, Vance G, Boersma B. HPLC-Mass Spectrometry of Isoflavonoids in Soy and the American Groundnut, Apios Americana. In: Buslig BS, Manthey JA, editors. Flavonoids in Cell Function. Kluwer Academic/Plenum Publishers; New York, NY: 2002. pp. 77–88. - PubMed
- Yazaki K, Sasaki K, Tsurumaru Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry. 2009;70:1739–1745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials