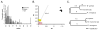Adaptive introgression of anticoagulant rodent poison resistance by hybridization between old world mice - PubMed (original) (raw)
Adaptive introgression of anticoagulant rodent poison resistance by hybridization between old world mice
Ying Song et al. Curr Biol. 2011.
Abstract
Polymorphisms in the vitamin K 2,3-epoxide reductase subcomponent 1 (vkorc1) of house mice (Mus musculus domesticus) can cause resistance to anticoagulant rodenticides such as warfarin [1-3]. Here we show that resistant house mice can also originate from selection on vkorc1 polymorphisms acquired from the Algerian mouse (M. spretus) through introgressive hybridization. We report on a polymorphic introgressed genomic region in European M. m. domesticus that stems from M. spretus, spans >10 Mb on chromosome 7, and includes the molecular target of anticoagulants vkorc1 [1-4]. We show that in the laboratory, the homozygous complete vkorc1 allele of M. spretus confers resistance when introgressed into M. m. domesticus. Consistent with selection on the introgressed allele after the introduction of rodenticides in the 1950s, we found signatures of selection in patterns of variation in M. m. domesticus. Furthermore, we detected adaptive protein evolution of vkorc1 in M. spretus (Ka/Ks = 1.54-1.93) resulting in radical amino acid substitutions that apparently cause anticoagulant tolerance in M. spretus as a pleiotropic effect. Thus, positive selection produced an adaptive, divergent, and pleiotropic vkorc1 allele in the donor species, M. spretus, which crossed a species barrier and produced an adaptive polymorphic trait in the recipient species, M. m. domesticus.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Figure 1
A) Shown are the variable positions defining 20 vkorc1 genotypes identified in the transcribed sequences of 106 Western European house mice (M. musculus domesticus) (no variants were detected in 5′ UTR). DNA Sanger-sequencing and alignments to vkorc1 of the Algerian mouse (M. spretus) were done. Intron sequences were determined whenever possible (i.e. not obscured by insertion/deletion polymorphisms) and used to infer whether ambiguous transcript sequences were of M. m. domesticus origin or were M. spretus. M. m. domesticus C57BL/6J and M. spretus shown on the top and bottom, respectively. We define genotypes as vkorc1dom if no traces of any M. spretus polymorphisms could be detected in the coding and non-coding regions of the gene (genotypes 1–6). The vkorc1 genotypes that correspond to the vkorc1 sequence of M. spretus fully (genotype 20), or contain any discernible M. spretus variants either in form of heterozygosity and/or intragenic recombination in any part of the coding and non-coding portion of the gene (genotypes 8–19) are defined as vkorc1spr. Ambiguous genotypes that contain vkorc1 of M. m. domesticus (exons 1–2) but could not be assigned to either species in the 3 prime portion of the gene (green, pooled as genotype 7). Dots depict nucleotide states identical to C57BL/6J. Hyphens depict insertions/deletions. Empty fields depict missing information (but confirmed as either vkorc1dom or vkorc1spr based on flanking non-coding sequences [16]), and ‘?’ depict missing information that could not be assigned to either species based on flanking sequences. The standard nucleotide ambiguity and amino acid one-letter codes apply. Countries and genotype counts are shown in the right panel (n.a.–not applicable). Populations Spain-1 and -2 are listed separately because we analyzed these in more detail (see text). Asterisks mark those amino-acid variants and positions present in M. musculus and known to affect warfarin tolerance in rodents or humans [2, 3] (Y139C/S variants were not detected by us). The newly discovered W59L SNP affects a position in vkorc1 known to alter warfarin tolerance in form of a W59R non-synonymous SNP in Rattus norvegicus [2]. B) Distribution of vkorc1 genotypes in Western European M. m. domesticus. The hatched area depicts the native range of M. spretus [12]. The house mouse has become a cosmopolitan species and now is occurring across the entire area depicted and beyond [29]. Pie charts show the frequencies of pure vkorc1 of M. m. domesticus origin (vkorc1dom) (pink, genotypes 1–6 in A), genotypes that correspond to the complete M. spretus vkorc1 allele or share parts of it in form of heterozygosity and/or intragenic recombination (all vkorc1spr, yellow, genotypes 8–20 in A). Countries sampled are shaded in grey. Sampling locations (some overlapping due to proximity) are shown as triangles (pink–vkorc1spr absent, yellow - vkorc1spr present). C.f. Table S1 for sample information and Table S2 for PCR and sequencing primers.
Figure 2
Genome profiling of 10 M. m. domesticus from Germany and Spain. A. Coverage of genes, their transcript orientation and chromosomal physical positions (in megabases; Mb) (c.f. Table S2 for gene and PCR/sequencing primer information). B. VISTA plot depicting pairwise DNA sequence similarity scores (Y-axes, right, scaled between 90–100%) between C57BL/6J and 6 M. m. domesticus from Germany with genomic profiles I–VI and 4 from Spain (genome profiles VII–X). Exons are shown in purple, the coloring scheme is as in Figs. 1–2 indicating, at a coarse resolution; regions comprised out of predominantly M. m. domesticus sequences (pink) and M. spretus sequences (yellow). C. Minimum number of recombination events (black diamonds) within chromosome 7 among M. m. domesticus (excluding M. spretus and C57BL/6J). See also the analysis of linkage disequilibrium in Fig. S1B. D. Gene genealogies of M. m. domesticus identified as monophyletic or paraphyletic with respect to M. spretus, using 90% support for nodes as cutoff (c.f. Figure S1C and D). Significance of topologies is given in percent bootstrap values supporting monophyly of M. m. domesticus samples (top) or both clusters in paraphyletic topologies (bottom; first number M. m. domesticus, second number M. spretus). E. Plot of polymorphism in M. m. domesticus relative to divergence to M. spretus. F. Asterisks mark significance (at α=0.05, 0.01, and 0.001) of rejection of Hudson-Kreitman-Aquade (HKA) testing done on select non-recombining segments representing reference genes (grey shaded boxes; c.f. A for gene identifiers).
Figure 3
Adaptive evolution of vkorc1 in the M. spretus lineage. A. Plot of Ka/Ks between M. m. domesticus and M. spretus 184 gene transcripts. To reflect our confidence in orthology transcripts are grouped in order of decreasing confidence in orthology as one2one - one Inparanoid hit in each species; n2one - one hit in one species but many hits in the other; n2m - multiple hits in both species. The positions of vkorc1 (minimum and maximum Ka/Ks) in this distribution are shown as *. B. Plot of Ks versus Ka of vkorc1 between R. norvegicus and M. spretus and M. musculus spp. (M. m. domesticus, M. m. musculus, M. m. castaneus, M. m. molossinus) (black triangles), between members of M. musculus spp. (pink diamonds), and between M. musculus spp. and M. spretus (yellow squares). The dashed line depicts Ka=Ks expected under selective neutrality. C. Mapping of non-synonymous substitutions on the vkorc1 neighbor-joining phylogeny of M. musculus spp., M. spretus, and R. norvegicus. Numbers above branches indicate the average number of nucleotide substitutions. A significant excess of non-synonymous substitutions (_n_=4), as determined using Tajima’s relative rate test, is indicated by*
Comment in
- Adaptive introgression: the seeds of resistance.
Rieseberg L. Rieseberg L. Curr Biol. 2011 Aug 9;21(15):R581-3. doi: 10.1016/j.cub.2011.06.038. Curr Biol. 2011. PMID: 21820620
Similar articles
- Bidirectional Introgression between Mus musculus domesticus and Mus spretus.
Banker SE, Bonhomme F, Nachman MW. Banker SE, et al. Genome Biol Evol. 2022 Jan 4;14(1):evab288. doi: 10.1093/gbe/evab288. Genome Biol Evol. 2022. PMID: 35038727 Free PMC article. - Study of the efficiency of anticoagulant rodenticides to control Mus musculus domesticus introgressed with Mus spretus Vkorc1.
Goulois J, Hascoët C, Dorani K, Besse S, Legros L, Benoit E, Lattard V. Goulois J, et al. Pest Manag Sci. 2017 Feb;73(2):325-331. doi: 10.1002/ps.4319. Epub 2016 Jun 23. Pest Manag Sci. 2017. PMID: 27196872 - Mus musculus populations in Western Australia lack VKORC1 mutations conferring resistance to first generation anticoagulant rodenticides: Implications for conservation and biosecurity.
Duncan BJML, Koenders A, Burnham Q, Lohr MT. Duncan BJML, et al. PLoS One. 2020 Sep 24;15(9):e0236234. doi: 10.1371/journal.pone.0236234. eCollection 2020. PLoS One. 2020. PMID: 32970676 Free PMC article. - [Vitamin K epoxide reductase: Fresh blood for oral anticoagulant therapies].
Loriot MA, Beaune P. Loriot MA, et al. Rev Med Interne. 2006 Dec;27(12):979-82. doi: 10.1016/j.revmed.2006.09.004. Epub 2006 Oct 11. Rev Med Interne. 2006. PMID: 17070618 Review. French. - Pharmacogenetics of oral anticoagulants: a basis for dose individualization.
Stehle S, Kirchheiner J, Lazar A, Fuhr U. Stehle S, et al. Clin Pharmacokinet. 2008;47(9):565-94. doi: 10.2165/00003088-200847090-00002. Clin Pharmacokinet. 2008. PMID: 18698879 Review.
Cited by
- Harmonizing hybridization dissonance in conservation.
Quilodrán CS, Montoya-Burgos JI, Currat M. Quilodrán CS, et al. Commun Biol. 2020 Jul 21;3(1):391. doi: 10.1038/s42003-020-1116-9. Commun Biol. 2020. PMID: 32694629 Free PMC article. Review. - Little or no gene flow despite F1 hybrids at two interspecific contact zones.
Mckean NE, Trewick SA, Morgan-Richards M. Mckean NE, et al. Ecol Evol. 2016 Mar 9;6(8):2390-404. doi: 10.1002/ece3.1942. eCollection 2016 Apr. Ecol Evol. 2016. PMID: 27066230 Free PMC article. - Multiple origins of melanism in two species of North American tree squirrel (Sciurus).
McRobie HR, Moncrief ND, Mundy NI. McRobie HR, et al. BMC Evol Biol. 2019 Jul 11;19(1):140. doi: 10.1186/s12862-019-1471-7. BMC Evol Biol. 2019. PMID: 31296164 Free PMC article. - Bidirectional Introgression between Mus musculus domesticus and Mus spretus.
Banker SE, Bonhomme F, Nachman MW. Banker SE, et al. Genome Biol Evol. 2022 Jan 4;14(1):evab288. doi: 10.1093/gbe/evab288. Genome Biol Evol. 2022. PMID: 35038727 Free PMC article. - Adaptive introgression between Anopheles sibling species eliminates a major genomic island but not reproductive isolation.
Clarkson CS, Weetman D, Essandoh J, Yawson AE, Maslen G, Manske M, Field SG, Webster M, Antão T, MacInnis B, Kwiatkowski D, Donnelly MJ. Clarkson CS, et al. Nat Commun. 2014 Jun 25;5:4248. doi: 10.1038/ncomms5248. Nat Commun. 2014. PMID: 24963649 Free PMC article.
References
- Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hortnagel K, Pelz HJ, Lappegard K, Seifried E, Scharrer I, Tuddenham EGD, Muller CR, Strom TM, Oldenburg J. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. - PubMed
- Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, Stafford DW. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427:541–544. - PubMed
- Scully MF. Warfarin therapy: rat poison and the prevention of thrombosis. The Biochemist. 2002;24:15–17.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous