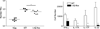Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses - PubMed (original) (raw)
Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses
Botond Z Igyártó et al. Immunity. 2011.
Abstract
Skin-resident dendritic cells (DCs) are well positioned to encounter cutaneous pathogens and are required for the initiation of adaptive immune responses. There are at least three subsets of skin DC- Langerhans cells (LC), Langerin(+) dermal DCs (dDCs), and classic dDCs. Whether these subsets have distinct or redundant function in vivo is poorly understood. Using a Candida albicans skin infection model, we have shown that direct presentation of antigen by LC is necessary and sufficient for the generation of antigen-specific T helper-17 (Th17) cells but not for the generation of cytotoxic lymphocytes (CTLs). In contrast, Langerin(+) dDCs are required for the generation of antigen specific CTL and Th1 cells. Langerin(+) dDCs also inhibited the ability of LCs and classic DCs to promote Th17 cell responses. This work demonstrates that skin-resident DC subsets promote distinct and opposing antigen-specific responses.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Figure 1. C. albicans skin infection model
Skin of wild-type mice were infected with 2 × 108 blastoconidia of the SC5314 strain of C. albicans. a) Skin from infected mice harvested on day+2 after infection was sectioned and stained with PAS to highlight fungi. Numerous yeast forms are evident on the external surface of the skin. Filamentous forms (arrow heads) can be observed penetrating through the epidermis into the dermis. Representative images are shown. Bar=50µm. b) Skin samples from cohorts of WT (solid line, n=8) and huLangerin-DTA (broken line, n=8) were cleaned with provine-iodine prior to being harvested at the indicated time after infection. C. albicans CFU is expressed as colonies per mg of tissue. c) WT, _Rag1_−/− and huLangerin-DTA mice were infected on the skin or by intra-dermal injection of 107 blastoconidia. DTH responses on day +7 were measured by specific footpad swelling 24 hours after injection of 107 heat killed C. albicans. Data is representative of 3 independent experiments with cohorts of at least 6 mice per group. *p<0.05.
Figure 2. Generation of recombinant C. albicans (Calb-Ag)
a) EGFP and the model T cell antigens, 2W1S, OVA323–339, OVA257–264, and I-Eα50–66 were inserted in frame into the genome of C. albicans (SC5314) at the C-terminus of the enolase gene. Expression GFP was confirmed by (b) direct visualization fluorescence of Calb-Ag blastoconidia and by (c) flow cytometry comparing Calb-Ag (solid line) with Calb-WT (Shaded). To confirm expression and availability of the T cell epitopes, Calb-WT (shaded) and Calb-Ag (solid line) were cultured with irradiated spleen cells, and CFSE-labeled CD90.1 congenic OT-I (d) or OT-II (e) cells. The amount of CFSE on CD90.1 gated cells T cells is shown after 3 days in culture. Data are representative of 3 independent experiments.
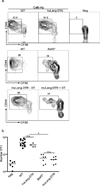
Figure 3. LC are not required for antigen cross presentation in vivo
a) 3×105 CFSE-labeled, CD90.1, CD44lo OT-I cells (CTL specific for OVA257–264) were adoptively transferred into WT, huLangerin-DTA (top panel), _Batf3_−/− (middle panel) or muLangerin-DTR (bottom panel) mice. Mice were infected on their skin with either Calb-WT (Neg) or Calb-Ag. Skin-draining LN were harvested 4 days later. CFSE and expression of CD44 are shown on CD8+, CD90.1+ gated cells. Numbers adjacent to outlined areas are the percentage of cells that have diluted CFSE. b) Total numbers of CD90.1+, OT-I cells recovered from each strain is shown. Each symbol represents an individual animal. WT and huLangerin-DTA are not significantly different. _Batf3_−/− and DT treated muLangerin-DTR mice are not significantly different from each other, but are significantly less than WT and huLangerin-DTA mice (p<0.0001). n.s.=not significant. See also Figure S1.
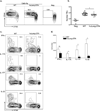
Figure 4. LC are necessary for Th17 cell development
3×105 CFSE-labeled, CD90.1 TEα cells (CD4+ T cells specific for I-Eα50–66) were transferred into WT and huLangerin-DTA mice. a) Mice were infected on their skin with either Calb-WT (Neg) or Calb-Ag. Skin-draining LN were harvested 4 days later. CFSE and expression of CD44 are shown on CD4+, CD90.1+ gated cells. b) Total numbers of CD90.1+, TEα cells recovered from each strain is shown. Each symbol represents an individual animal. c) As in (a) except that cells were re-stimulated i_n vitro_ with PMA-ionomycin prior to intracellular staining with the indicated cytokine antibody. Representative plots are shown. d) Total numbers of cytokine producing TEα cells from each strain is shown. Data have been pooled from 3 independent experiments (ns, not significant; * p<0.05). See also Figure S2.
Figure 5. Direct antigen presentation by LC is required for Th17 cell development
As in figure 4, TEα cells were adoptively transferred into WT and huLangerin-Cre x I-Aβ-flox mice prior to skin infection. a) The expansion of TEα cells in Calb-WT (neg) infected WT mice Calb-Ag infected WT and huLangerin-Cre x I-Aβ-flox is shown. Each symbol represents an individual animal. b) Total numbers of cytokine producing TEα cells from each strain is shown. Data have been pooled from 2 independent experiments (ns, not significant; * p<0.05).
Figure 6. Antigen presentation by LC is sufficient for Th17 development
a) WT (shaded) and huLangerin-DTR (solid line) mice were injected i.p. with 10 µg anti-huLangerin (2G3) conjugated to Alexa-647. The amount of Alexa-647 16 hours after injection is shown in epidermal cells gated on LC (CD45+, MHC-II+), keratinocytes (KC, CD45-, MHC-II-) and dendritic epidermal T cells (DETC, CD45+, MHC-II−). b) As in (a) except that LC isolated from the epidermis were identified by expression of endogenous mouse Langerin (green). The cellular location of 2G3-Alexa-647 (red) and colocalization with muLangerin (yellow) was visualized by immunofluorescence. c) As in (a), cells were isolated from skin-draining LN. The amount of 2G3-Alexa647 on LC (MHC-IIhi, CD11 c+, CD8−, Langerin+, CD103−), Langerin+ dDC (MHC-IIhi, CD11c+, CD8−, Langerin+, CD103+), and Langerin− dDC (MHC-IIhi, CD11c+, CD8−, Langerin−) is shown. d) WT or huLangerin-DTR mice were adoptively transferred with 3×105 naïve OT-I cells 24 hours prior to i.p injection of 1.0 ug 2G3-OVA257–264 or isotype control (neg). Mice were then infected with Calb-WT on their skin. As a positive control, WT mice were immunized with 20 ug 2G3-OVA257–264 in CFA (pos). Skin-draining LN were harvested on day+4 and the total number of OT-I cells is shown. e) As in (d) except that TEα cells were transferred and mice were immunized with 2G3-Eα. f) CFSE and expression of IL-17A from TEα isolated from WT and huLangerin-DTR mice infected with Calb-WT or S. aureus are shown. Data are representative of 3 individual experiments. See also Figure S3 and S4.
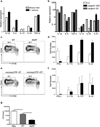
Figure 7. Langerin+ dDC promote Th1 and inhibit Th17 cell differentiation
a) LC were sorted by flow cytometry on day+4 from skin-draining LN from sham (open bars) or Calb-WT infected (black bars) muLangerin-EGFP reporter mice. Relative expression of mRNA of the indicated cytokines are shown. b) LC (open bars), Langerin+ dDC (black bars) and Langerin− dDC (gray bars) were sorted by flow cytometry from Calb-WT infected muLangerin-EGFP mice. Relative expression of mRNA of the indicated cytokines are shown. c and d) As in Figure 4, WT (open bars) and _Batf3_−/− (black bars) mice were infected with Calb-Ag. Proliferation (c) and cytokine expression (d) are shown. e and f) As in (c) except that muLangerin-DTR mice that had been injected with DT(black bars) or PBS (open bars) were used. Data are representative of 3 individual experiments. g) Cohorts of 4–8 WT and _Batf3_−/− mice were skin infected with Calb-WT or sham(“Neg”). Mice were then re-infected by intradermal injection of 5×106 Calb-WT on day +9. Three days later, skin was harvested and CFU obtained. (* p<0.05). Data are pooled from 2 independent experiments. See also Figure S5 and S6.
Similar articles
- Directly transfected langerin+ dermal dendritic cells potentiate CD8+ T cell responses following intradermal plasmid DNA immunization.
Elnekave M, Furmanov K, Nudel I, Arizon M, Clausen BE, Hovav AH. Elnekave M, et al. J Immunol. 2010 Sep 15;185(6):3463-71. doi: 10.4049/jimmunol.1001825. Epub 2010 Aug 16. J Immunol. 2010. PMID: 20713888 - Skin langerin+ dendritic cells transport intradermally injected anti-DEC-205 antibodies but are not essential for subsequent cytotoxic CD8+ T cell responses.
Flacher V, Tripp CH, Haid B, Kissenpfennig A, Malissen B, Stoitzner P, Idoyaga J, Romani N. Flacher V, et al. J Immunol. 2012 Mar 1;188(5):2146-55. doi: 10.4049/jimmunol.1004120. Epub 2012 Jan 30. J Immunol. 2012. PMID: 22291181 Free PMC article. - Langerin+ dermal dendritic cells are critical for CD8+ T cell activation and IgH γ-1 class switching in response to gene gun vaccines.
Stoecklinger A, Eticha TD, Mesdaghi M, Kissenpfennig A, Malissen B, Thalhamer J, Hammerl P. Stoecklinger A, et al. J Immunol. 2011 Feb 1;186(3):1377-83. doi: 10.4049/jimmunol.1002557. Epub 2010 Dec 27. J Immunol. 2011. PMID: 21187444 - Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells.
Merad M, Ginhoux F, Collin M. Merad M, et al. Nat Rev Immunol. 2008 Dec;8(12):935-47. doi: 10.1038/nri2455. Nat Rev Immunol. 2008. PMID: 19029989 Review. - Ontogeny and function of murine epidermal Langerhans cells.
Kaplan DH. Kaplan DH. Nat Immunol. 2017 Sep 19;18(10):1068-1075. doi: 10.1038/ni.3815. Nat Immunol. 2017. PMID: 28926543 Free PMC article. Review.
Cited by
- Recording of DNA-binding events reveals the importance of a repurposed Candida albicans regulatory network for gut commensalism.
Witchley JN, Basso P, Brimacombe CA, Abon NV, Noble SM. Witchley JN, et al. Cell Host Microbe. 2021 Jun 9;29(6):1002-1013.e9. doi: 10.1016/j.chom.2021.03.019. Epub 2021 Apr 28. Cell Host Microbe. 2021. PMID: 33915113 Free PMC article. - Signaling through IL-17C/IL-17RE is dispensable for immunity to systemic, oral and cutaneous candidiasis.
Conti HR, Whibley N, Coleman BM, Garg AV, Jaycox JR, Gaffen SL. Conti HR, et al. PLoS One. 2015 Apr 7;10(4):e0122807. doi: 10.1371/journal.pone.0122807. eCollection 2015. PLoS One. 2015. PMID: 25849644 Free PMC article. - Functional Specialty of CD40 and Dendritic Cell Surface Lectins for Exogenous Antigen Presentation to CD8(+) and CD4(+) T Cells.
Yin W, Gorvel L, Zurawski S, Li D, Ni L, Duluc D, Upchurch K, Kim J, Gu C, Ouedraogo R, Wang Z, Xue Y, Joo H, Gorvel JP, Zurawski G, Oh S. Yin W, et al. EBioMedicine. 2016 Jan 28;5:46-58. doi: 10.1016/j.ebiom.2016.01.029. eCollection 2016 Mar. EBioMedicine. 2016. PMID: 27077111 Free PMC article. - Regulation of the Migration of Distinct Dendritic Cell Subsets.
Feng M, Zhou S, Yu Y, Su Q, Li X, Lin W. Feng M, et al. Front Cell Dev Biol. 2021 Feb 19;9:635221. doi: 10.3389/fcell.2021.635221. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33681216 Free PMC article. Review. - Extrathymic expression of Aire controls the induction of effective TH17 cell-mediated immune response to Candida albicans.
Dobeš J, Ben-Nun O, Binyamin A, Stoler-Barak L, Oftedal BE, Goldfarb Y, Kadouri N, Gruper Y, Givony T, Zalayat I, Kováčová K, Böhmová H, Valter E, Shulman Z, Filipp D, Husebye ES, Abramson J. Dobeš J, et al. Nat Immunol. 2022 Jul;23(7):1098-1108. doi: 10.1038/s41590-022-01247-6. Epub 2022 Jun 27. Nat Immunol. 2022. PMID: 35761088
References
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. - PubMed
- Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M. TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur J Immunol. 2009;39:1221–1230. - PubMed
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. - PubMed
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI062427/AI/NIAID NIH HHS/United States
- R01 AR056632/AR/NIAMS NIH HHS/United States
- U19 AI057234/AI/NIAID NIH HHS/United States
- R01-AR056632/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases