Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway - PubMed (original) (raw)
Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway
Qingyuan Meng et al. J Biol Chem. 2011.
Abstract
Autophagy has been recently demonstrated to control cell and tissue homeostasis, including the functions of various metabolic tissues. However, it remains unclear whether autophagy is critical for the central nervous system and particularly the hypothalamus for exerting metabolic regulation. Using autophagy-related protein 7 (Atg7) as an autophagic marker, this work showed that autophagy was highly active in the mediobasal hypothalamus of normal mice. In contrast, chronic development of dietary obesity was associated with autophagic decline in the mediobasal hypothalamus. To investigate the potential role of autophagy in the hypothalamic control of metabolic physiology, a mouse model was developed with autophagic inhibition in the mediobasal hypothalamus using site-specific delivery of lentiviral shRNA against Atg7. This model revealed that hypothalamic inhibition of autophagy increased energy intake and reduced energy expenditure. These metabolic changes were sufficient to increase body weight gain under normal chow feeding and exacerbate the progression of obesity and whole-body insulin resistance under high-fat diet feeding. To explore the underlying mechanism, this study found that defective hypothalamic autophagy led to hypothalamic inflammation, including the activation of proinflammatory IκB kinase β pathway. Using brain-specific IκB kinase β knockout mice, it was found that the effects of defective hypothalamic autophagy in promoting obesity were reversed by IκB kinase β inhibition in the brain. In conclusion, hypothalamic autophagy is crucial for the central control of feeding, energy, and body weight balance. Conversely, decline of hypothalamic autophagy under conditions of chronic caloric excess promotes hypothalamic inflammation and thus impairs hypothalamic control of energy balance, leading to accelerated development of obesity and comorbidities.
Figures
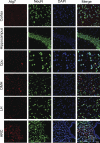
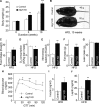
FIGURE 1.
Distribution of Atg7 in the brain and hypothalamus. Coimmunostaining of Atg7 (red) with neuronal marker NeuN (green) was performed for the sections across various brain areas and hypothalamic regions of normal C57BL/6 mice (chow-fed adult males). DAPI staining (blue) reveals the nucleus of all cells in the sections. Images were merged to show Atg-immunoreactive cells among the total cell population in the sections. Data show representative immunostaining in the cortex, hippocampus, caudate putamen (Cpu), dorsal medial hypothalamus (DMH), lateral hypothalamus (LH), and arcuate nucleus (ARC). At least three mice per brain regions were analyzed. Scale bar = 100 μm.
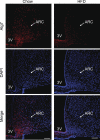
FIGURE 2.
Effect of HFD feeding on Atg7 in hypothalamic arcuate nucleus. Male C57BL/6 mice were maintained on a normal chow diet versus an HFD, and brain sections were then prepared for Atg7 immunostaining (red). Data show Atg7 immunostaining across the hypothalamic arcuate nucleus (ARC) of chow-fed versus HFD-fed C57BL/6 mice. DAPI staining (blue) reveals the nucleus of all cells in the sections. At least five mice per group were analyzed. Data represent approximately 4–5 months of HFD versus chow feeding. 3V, third ventricle. Scale bar = 100 μm.
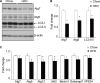
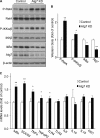
FIGURE 3.
Effects of HFD feeding on autophagy markers in the hypothalamus. Male C57BL/6 mice were maintained on a normal chow diet versus an HFD. The hypothalamus was harvested for the measurements of the indicated autophagic markers by Western blot analyses (A and B) and real-time RT-PCR analyses (C). Quantification of Western blot analyses are presented in B. LC3-II/I, ratio of LC3-II to LC3-I. *, p < 0.05; n = 3 (B) and 10 (C) per group. Data are presented as mean ± S.E.
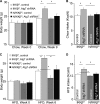
FIGURE 4.
Metabolic phenotype of mice with MBH-specific Atg7 knockdown on chow feeding. C57BL/6 mice (chow-fed adult males) with Atg7 knockdown (Atg7 KD) versus the control were generated using bilateral injections of the MBH with lentiviruses containing shRNA against mouse Atg7 or the matched control shRNA. A, Atg7 immunostaining (red) of brain sections was performed to verify Atg7 knockdown in the MBH. DAPI staining (blue) reveals the nuclei of all cells in the sections. Scale bar = 50 μm. KD, knockdown. B and C, longitudinal follow-up of body weight (B) and average daily food intake (C) of chow-fed mice over 12 weeks post-MBH injection. *, p < 0.05; **, p < 0.01 (comparisons between two mouse groups at the matched time points that are underlined); n = 12–13 per group. Data are presented as mean ± S.E. D, metabolic chamber assessment of O2 consumption was performed for a subgroup of chow-fed mice at week 5 post injection. O2 consumption was corrected by lean body mass of mice. *, p < 0.05; n = 4 per group. Data are presented as mean ± S.E. E, net energy balance was calculated by subtracting energy expenditure ((3.815 + 1.232 × VCO2/VO2) × VO2, Columbus Instruments) from energy intake for mice at week 5 post-injection. *, p < 0.05; n = 4 per group. Data are presented as mean ± S.E. F and G, MRI assessment of fat mass (D) and lean mass (E) was performed for a subgroup of chow-fed mice at 5 weeks post-injection. *, p < 0.05; n = 6–8 per group. Data are shown as mean ± S.E. H–J, a subgroup of chow-fed mice was analyzed for glucose tolerance (H), fasting blood insulin concentration (I), and fasting blood leptin concentration (J) at week 10 post-injection. *, p < 0.05; n = 6 per group. Data are shown as mean ± S.E. GTT, glucose tolerance test; Con, control.
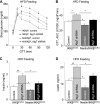
FIGURE 5.
Metabolic phenotype of mice with MBH-specific Atg7 knockdown under HFD feeding. Body weight-matched C57BL/6 mice (chow-fed adult males) with Atg7 knockdown (Atg7 KD) versus the control were generated using bilateral injections of the MBH with lentiviruses containing shRNA against mouse Atg7 or the matched control shRNA. Mice were switched to HFD feeding post-lentiviral injection. A–C, longitudinal follow-up of body weight (A), obesity appearance (B), and average daily food intake (C) of HFD-fed mice at the indicated time points post-MBH injection. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n = 10 - 12 per group. Data are presented as mean ± S.E. KD, knockdown. D, metabolic chamber assessment of energy expenditure was performed for a subgroup of HFD-fed mice at week 3 post-injection. *, p < 0.05; n = 4 per group. Data are presented as mean ± S.E. E, net energy balance was calculated by subtracting energy expenditure ((3.815 + 1.232 × VCO2/VO2) × VO2, Columbus Instruments) from energy intake for mice at week 3 post-injection. **, p < 0.01; n = 4 per group. Data are presented as mean ± S.E. F and G, MRI assessment of fat mass (F) and lean mass (G) was performed at 3 weeks post-injection. **, p < 0.01; n = 12 per group. Data are shown as mean ± S.E. H–J, mice were analyzed with a glucose tolerance at week 4 post-injection (H) and for fasting blood insulin (I) and leptin (J) concentrations at week 10 post-injection. *, p < 0.05; **, p < 0.01; n = 6–12 per group. Data are shown as mean ± S.E. GTT, glucose tolerance test.
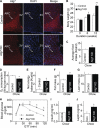
FIGURE 6.
Effects of Atg7 knockdown on IKKβ/NF-κB and related inflammation in the hypothalamus. C57BL/6 mice (chow-fed adult males) with Atg7 knockdown (Atg7 KD) versus the control were generated using bilateral injections of the MBH with lentiviruses containing Atg7 shRNA or matched control shRNA. Hypothalami were harvested at 1 week post-injection and analyzed for IKKβ/NF-κB signaling by Western blotting (A and B) and for gene expression of related inflammatory molecules by real-time RT-PCR (C). P-RelA, phosphorylated RelA; _P-IKK_α/β, phosphorylated IKKα/β. The bar graphs in B show the quantification analysis of Western blot analyses. *, p < 0.05; **, p < 0.01; n = 3 per group (B) and 6 per group (C). Data are shown as mean ± S.E.
FIGURE 7.
IKKβ ablation in the brain prevents the metabolic effects of MBH-Atg7 knockdown. Nestin/IKKβlox/lox mice (N/IKK_β_l/l) and littermate controls (IKKβlox/lox mice, IKK_β_l/l) were maintained under chow feeding since weaning. At an adult age, mice were bilaterally injected with lentivirus to deliver Atg7 shRNA or control shRNA to the MBH. Following injection, mice were maintained on either chow feeding (A and B) or HFD feeding (C and D). The longitudinal body weight (A and C) and daily food intake (B and D) of these mice were followed up. *, p < 0.05; n = 6–8 per group. Data are presented as mean ± S.E.
FIGURE 8.
IKKβ ablation in the brain prevents the metabolic effects of MBH-Atg7 knockdown. Nestin/IKKβlox/lox mice (N/IKK_β_l/l) and littermate controls (IKKβlox/lox mice, IKK_β_l/l) were maintained under chow feeding since weaning. At an adult age, mice with matched body weight were bilaterally injected with lentivirus to deliver either Atg7 shRNA or control shRNA to the MBH. Following injection, mice were maintained on HFD feeding. Mice were analyzed for glucose tolerance (A and B), fasting blood insulin (C), and leptin (D) concentrations at week 6 post-injection. The bar graphs in B show the area under curve (AUC) values of the glucose tolerance test (GTT) presented in A. *, p < 0.05; **, p < 0.01; n = 6–8 per group. Data are presented as mean ± S.E.
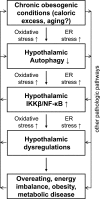
FIGURE 9.
Model for hypothalamic defect of autophagy in the pathogenesis of obesity and related disease. Chronic obesogenic conditions (including caloric excess and possibly aging) can reduce the function of hypothalamic autophagy, leading to IKKβ/NF-κB activation and inflammation in the hypothalamus. As a result, the hypothalamic function of regulating body weight and metabolic homeostasis is impaired, which potentiates the development of obesity and related metabolic diseases. In conjunction with the literature, oxidative stress and ER stress are likely involved in causing hypothalamic defect of autophagy, and, on the other hand, the onset of defective hypothalamic autophagy is predicted to also promote these intracellular stresses. In sum, all these changes can sustain IKKβ/NF-κB activation and related inflammation in the hypothalamus and thereby exacerbate the progression of obesity and related metabolic disease.
Similar articles
- Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation.
Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP. Douglass JD, et al. Mol Metab. 2017 Jan 28;6(4):366-373. doi: 10.1016/j.molmet.2017.01.010. eCollection 2017 Apr. Mol Metab. 2017. PMID: 28377875 Free PMC article. - TGF-β1 down-regulation in the mediobasal hypothalamus attenuates hypothalamic inflammation and protects against diet-induced obesity.
Mendes NF, Gaspar JM, Lima-Júnior JC, Donato J Jr, Velloso LA, Araújo EP. Mendes NF, et al. Metabolism. 2018 Aug;85:171-182. doi: 10.1016/j.metabol.2018.04.005. Epub 2018 Apr 13. Metabolism. 2018. PMID: 29660453 - Hypothalamic inflammation in obesity and metabolic disease.
Jais A, Brüning JC. Jais A, et al. J Clin Invest. 2017 Jan 3;127(1):24-32. doi: 10.1172/JCI88878. Epub 2017 Jan 3. J Clin Invest. 2017. PMID: 28045396 Free PMC article. Review. - Central inhibition of IKKβ/NF-κB signaling attenuates high-fat diet-induced obesity and glucose intolerance.
Benzler J, Ganjam GK, Pretz D, Oelkrug R, Koch CE, Legler K, Stöhr S, Culmsee C, Williams LM, Tups A. Benzler J, et al. Diabetes. 2015 Jun;64(6):2015-27. doi: 10.2337/db14-0093. Epub 2015 Jan 27. Diabetes. 2015. PMID: 25626735 - Hypothalamic dysfunction in obesity.
Williams LM. Williams LM. Proc Nutr Soc. 2012 Nov;71(4):521-33. doi: 10.1017/S002966511200078X. Epub 2012 Sep 6. Proc Nutr Soc. 2012. PMID: 22954151 Review.
Cited by
- Lipids, lysosomes, and autophagy.
Jaishy B, Abel ED. Jaishy B, et al. J Lipid Res. 2016 Sep;57(9):1619-35. doi: 10.1194/jlr.R067520. Epub 2016 Jun 21. J Lipid Res. 2016. PMID: 27330054 Free PMC article. Review. - Age-Associated Weight Gain, Leptin, and SIRT1: A Possible Role for Hypothalamic SIRT1 in the Prevention of Weight Gain and Aging through Modulation of Leptin Sensitivity.
Sasaki T. Sasaki T. Front Endocrinol (Lausanne). 2015 Jul 16;6:109. doi: 10.3389/fendo.2015.00109. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26236282 Free PMC article. Review. - Saturated fatty acids modulate autophagy's proteins in the hypothalamus.
Portovedo M, Ignacio-Souza LM, Bombassaro B, Coope A, Reginato A, Razolli DS, Torsoni MA, Torsoni AS, Leal RF, Velloso LA, Milanski M. Portovedo M, et al. PLoS One. 2015 Mar 18;10(3):e0119850. doi: 10.1371/journal.pone.0119850. eCollection 2015. PLoS One. 2015. PMID: 25786112 Free PMC article. - Leptin signaling and leptin resistance.
Zhou Y, Rui L. Zhou Y, et al. Front Med. 2013 Jun;7(2):207-22. doi: 10.1007/s11684-013-0263-5. Epub 2013 Apr 12. Front Med. 2013. PMID: 23580174 Free PMC article. Review. - Ginseng gintonin, aging societies, and geriatric brain diseases.
Choi SH, Lee R, Nam SM, Kim DG, Cho IH, Kim HC, Cho Y, Rhim H, Nah SY. Choi SH, et al. Integr Med Res. 2021 Mar;10(1):100450. doi: 10.1016/j.imr.2020.100450. Epub 2020 Jun 13. Integr Med Res. 2021. PMID: 32817818 Free PMC article. Review.
References
- Levine B. (2005) Cell 120, 159–162 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases